LIMMUNOTERAPIA ALLERGENE SPECIFICA AIT ATTUALE Giovanni Passalacqua Allergy
























- Slides: 56

L’IMMUNOTERAPIA ALLERGENE SPECIFICA (AIT) ATTUALE Giovanni Passalacqua Allergy & Respiratory Diseases Dept. Internal Medicine. University of Genoa ITALY

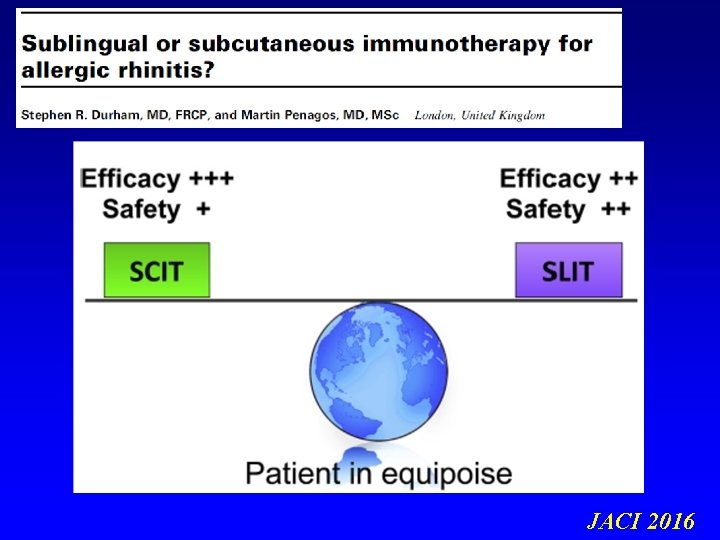
IMMUNOTERAPIA SPECIFICA (AIT) Somministrazione di estratti allergenici purificati (prima a dosi crescenti e poi a dose di mantenimento), al fine di ottenere la riduzione della risposta clinica all’allergene stesso. L’immunoterapia allergene specifica è un vaccino a tutti gli effetti La via tradizionale di somministrazione è quella iniettiva sottocutanea (SCIT), ad oggi affiancata anche dalla via sublinguale (SLIT)

Immunol Allergy Clin North Am. 2016

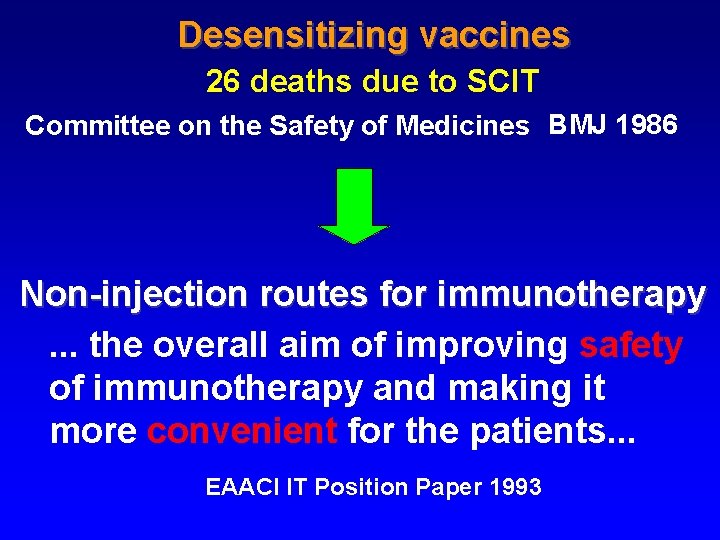
Committee on the safety of medicines (CMS) CMS Update Desensitizing vaccines Br Med J 1986; 293: 948 26 fatalities since 1957 certainly due to IT 11 of them since 1980

Dal 1910 fino agli anni ’ 70: Prescrizione ingiustificata dell’ITS Prescrizione non corretta Pratica non adeguata, senza regole precauzionali e con estratti scadenti DUBBIA EFFICACIA E SCARSA SICUREZZA

Desensitizing vaccines 26 deaths due to SCIT Committee on the Safety of Medicines BMJ 1986 Non-injection routes for immunotherapy. . . the overall aim of improving safety of immunotherapy and making it more convenient for the patients. . . EAACI IT Position Paper 1993

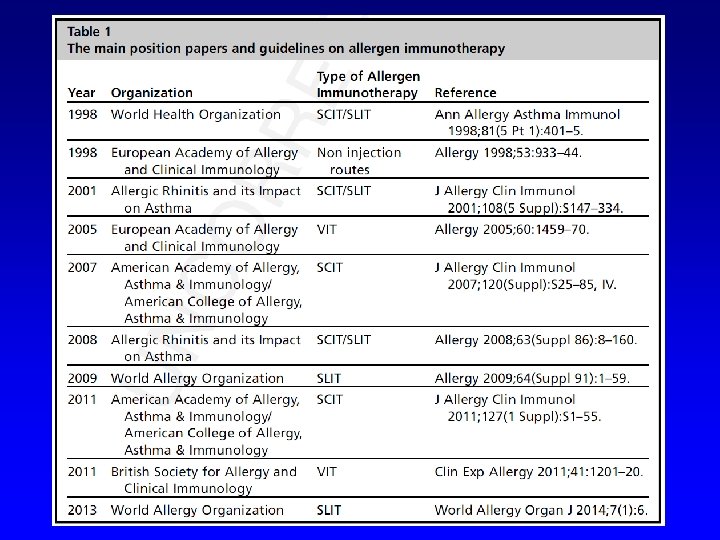
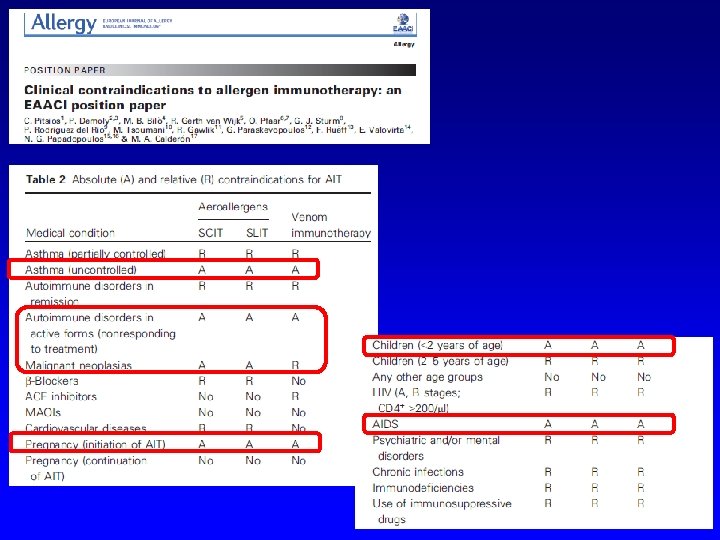
WHO Pos Pap. Therapeutical vaccines for allergic diseases Allergy 1998 Standards for practical allergen-specific immunotherapy. Allergy 2006 Allergen immunotherapy: A practice parameter third update JACI 2011


L'ITS e' mirata all'allergene causale e non all'organo principalmente coinvolto. ” L’ITS non è un trattamento di ultima scelta da usare se i farmaci falliscono, ma è complementare ad essi. L’ITS è efficace nelle allergie da - Inalanti (acari, pollini, alcuni funghi, epitelio di gatto) - Veleno di imenotteri

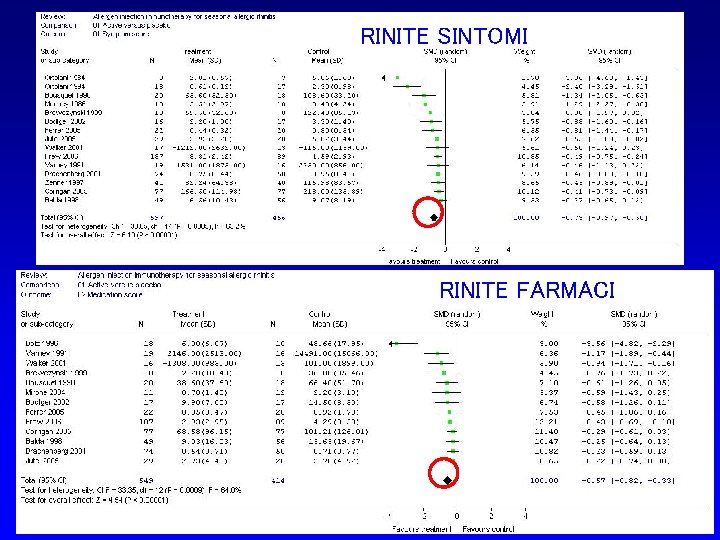
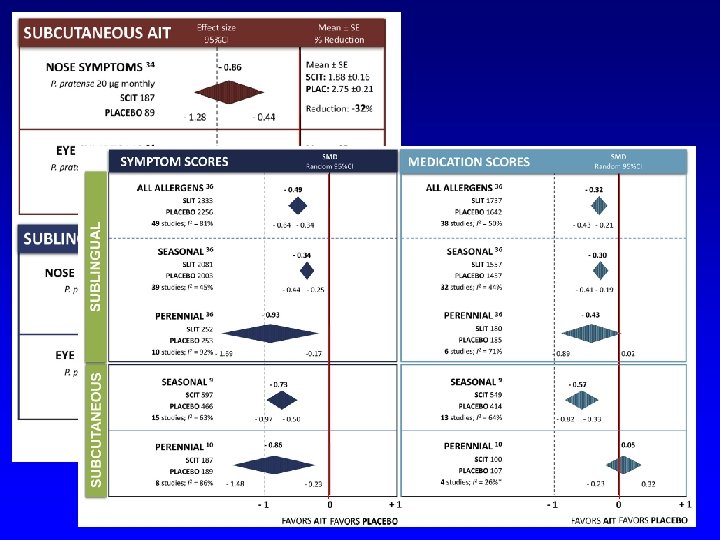
RINITE SINTOMI SCIT - Meta-analysis: Symptom score RINITE FARMACI Calderon M et al 2007

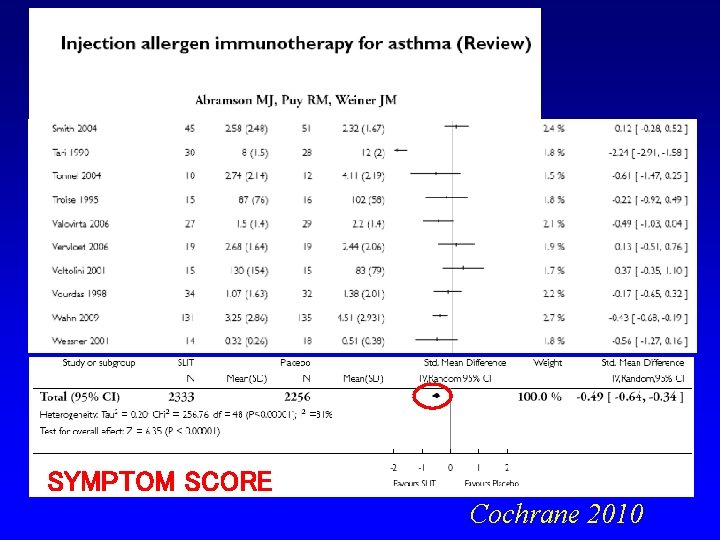
SYMPTOM SCORE Cochrane 2010

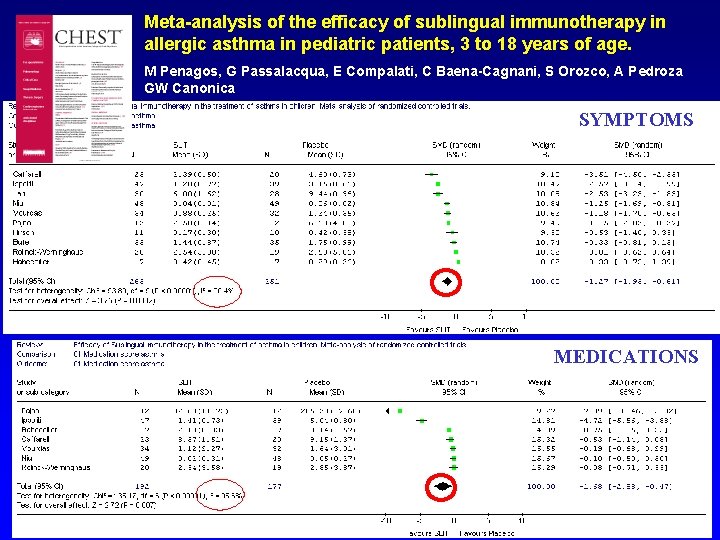
Meta-analysis of the efficacy of sublingual immunotherapy in allergic asthma in pediatric patients, 3 to 18 years of age. M Penagos, G Passalacqua, E Compalati, C Baena-Cagnani, S Orozco, A Pedroza GW Canonica SYMPTOMS MEDICATIONS

WHO pos pap (1998): 4 trials ARIA pos pap (2001): 22 trials EAACI pos pap (2006): 36 trials 1 st WAO pos pap (2009): 60 trials 2 nd WAO pos pap (2013): 77 trials After 2013: 87 trials

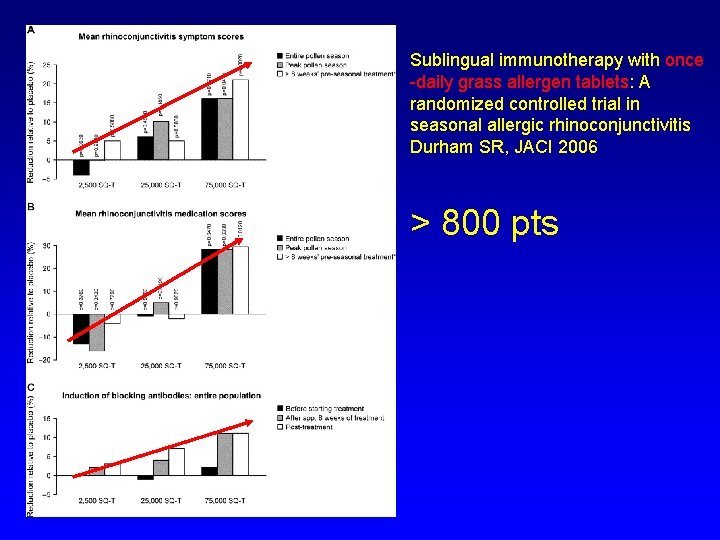
Sublingual immunotherapy with once -daily grass allergen tablets: A randomized controlled trial in seasonal allergic rhinoconjunctivitis Durham SR, JACI 2006 > 800 pts


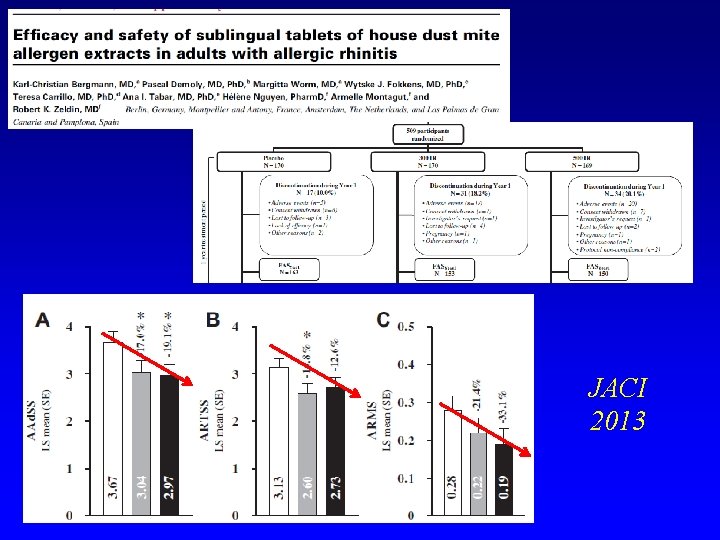
JACI 2013

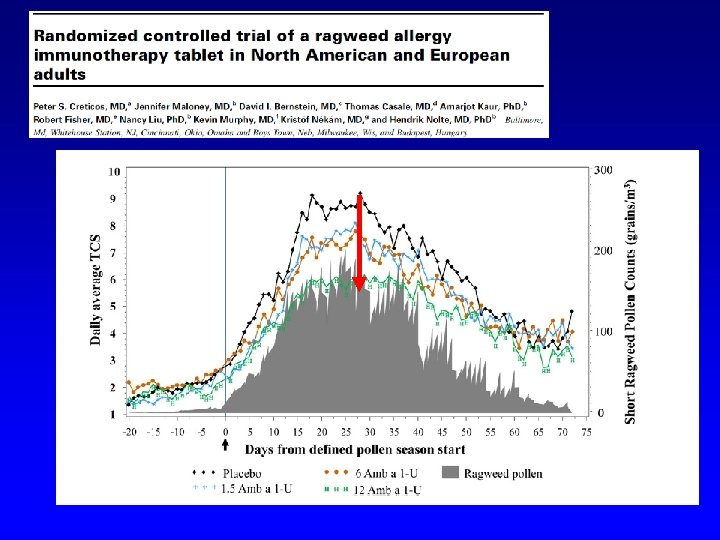
OPTIMAL DOSES (dose-finding studies) DURHAM 2006: 15 mcg Phl p 5/day DIDIER 2007: 25 mcg Group 5 /day CRETICOS 2013: 12 mcg Amb a 1/day BERGMANN 2014: 28/120 Der p 1/Der f 1/day MOSBECH 2014: 6 SQ/day (70 mcg day) NOLTE 2015: 12 DU/day (equivalent to 6 SQ/day)

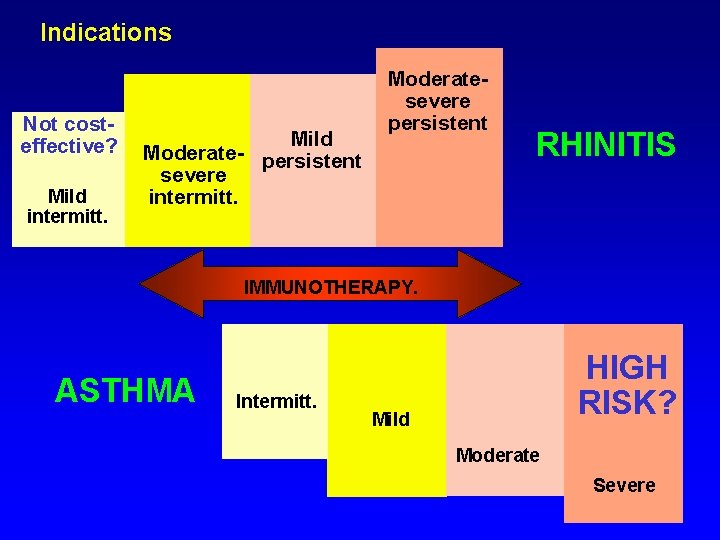
Indications Not costeffective? Mild intermitt. Mild Moderate- persistent severe intermitt. Moderatesevere persistent RHINITIS IMMUNOTHERAPY. ASTHMA Intermitt. HIGH RISK? Mild Moderate Severe

I fattori da valutare nella prescrizione dell’ITS 1 2 3 4 5 6 7 Il disturbo deve essere Ig. E - mediato (skin test o RAST positivi) L’allergene responsabile deve essere individuato con sicurezza Valutare la gravità e la durata dei sintomi l trattamento farmacologico é sufficientemente ben tollerato? Il paziente é in grado di affrontare l’ITS? (costi, impegno, stile di vita) È disponibile un vaccino standardizzato? L’efficacia del vaccino che si intende usare é dimostrata?

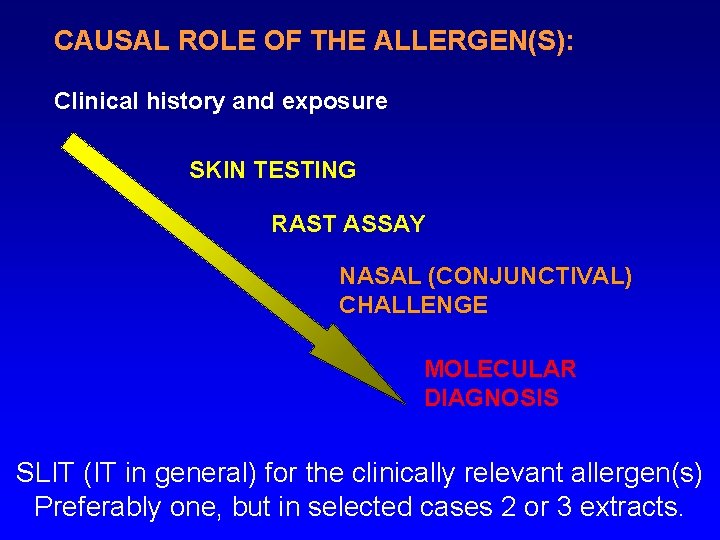
CAUSAL ROLE OF THE ALLERGEN(S): Clinical history and exposure SKIN TESTING RAST ASSAY NASAL (CONJUNCTIVAL) CHALLENGE MOLECULAR DIAGNOSIS SLIT (IT in general) for the clinically relevant allergen(s) Preferably one, but in selected cases 2 or 3 extracts.

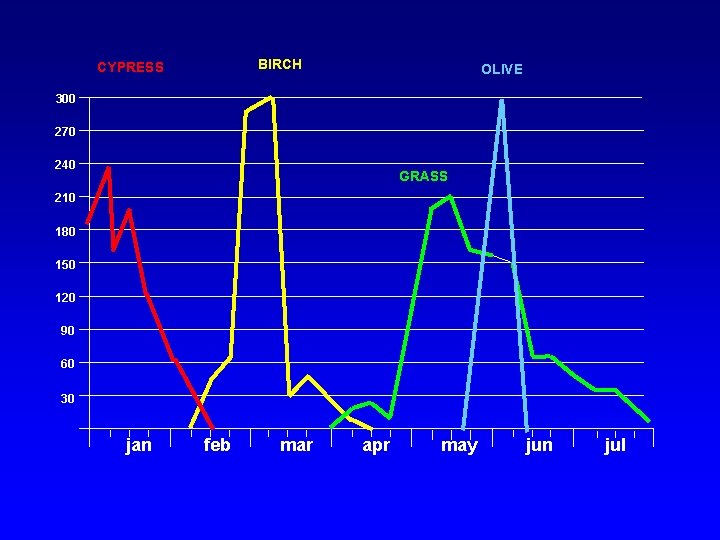
BIRCH CYPRESS OLIVE 300 270 240 GRASS 210 180 150 120 90 60 30 jan feb mar apr may jun jul

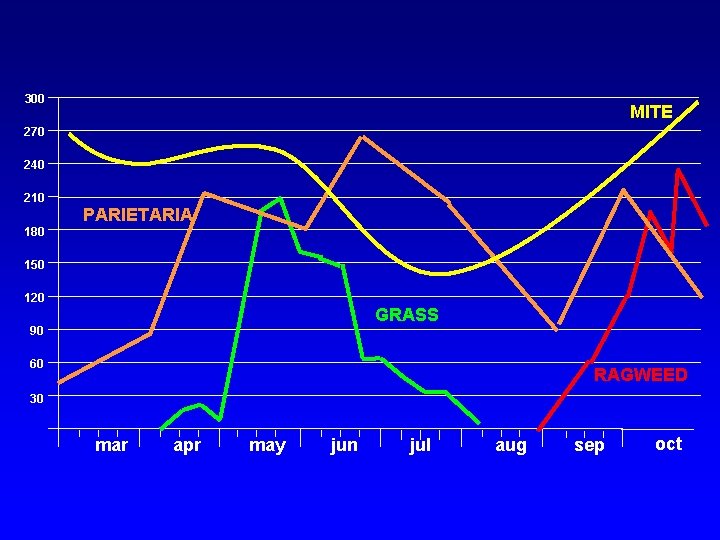
300 MITE 270 240 210 PARIETARIA 180 150 120 GRASS 90 60 RAGWEED 30 mar apr may jun jul aug sep oct

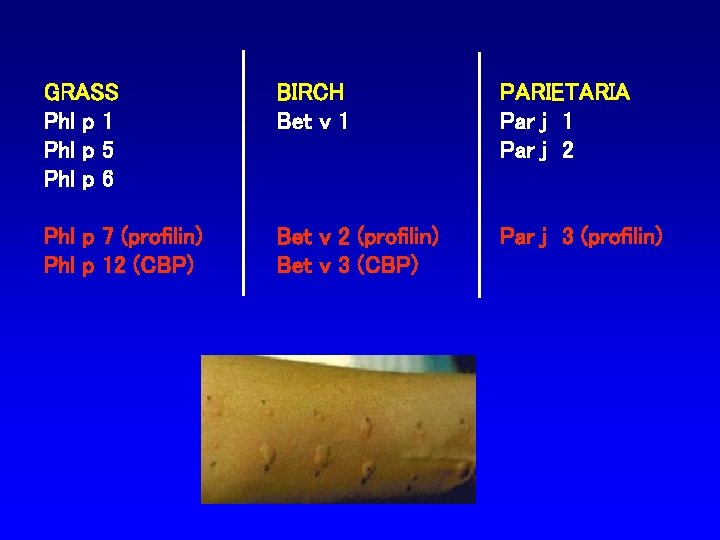
GRASS Phl p 1 Phl p 5 Phl p 6 BIRCH Bet v 1 PARIETARIA Par j 1 Par j 2 Phl p 7 (profilin) Phl p 12 (CBP) Bet v 2 (profilin) Bet v 3 (CBP) Par j 3 (profilin)

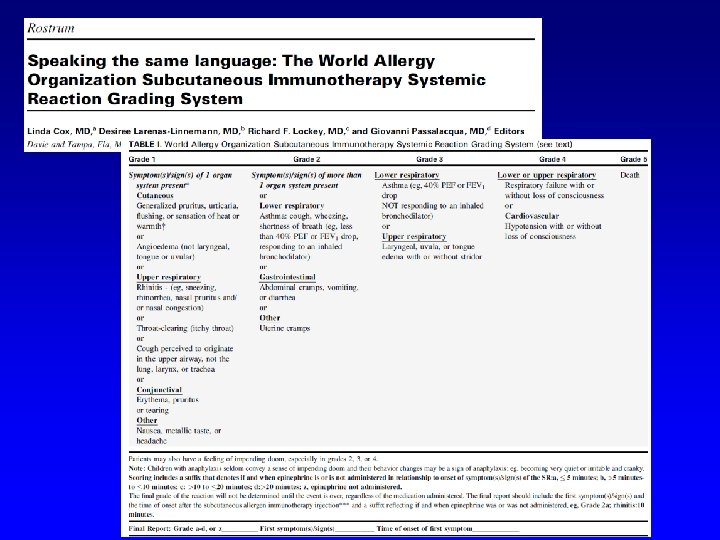
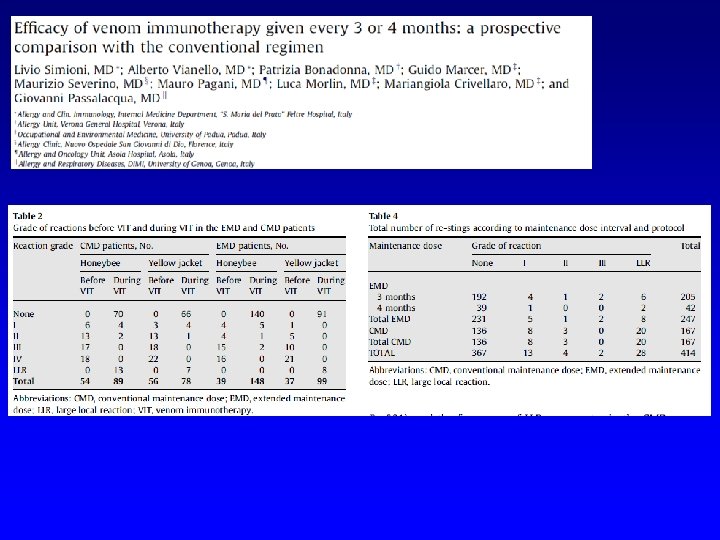
FATALITIES Lockey RF et al. JACI 1987 Period: 1945 -1984 46 fatalities Reid MJ et al. JACI 1993 Period 1985 -1989 17 fatalities FATALITIES: 1/2. 000 injections

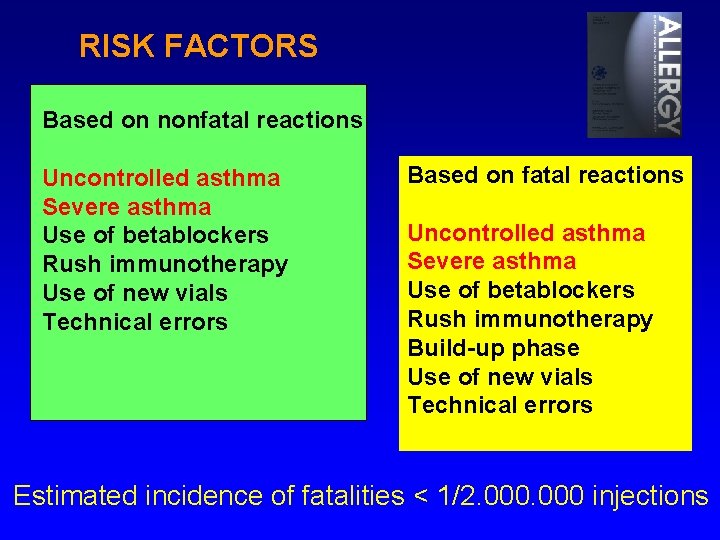
RISK FACTORS Based on nonfatal reactions Uncontrolled asthma Severe asthma Use of betablockers Rush immunotherapy Use of new vials Technical errors Based on fatal reactions Uncontrolled asthma Severe asthma Use of betablockers Rush immunotherapy Build-up phase Use of new vials Technical errors Estimated incidence of fatalities < 1/2. 000 injections

SEVERE ASTHMA (Control) NEAR FATAL ASTHMA (respiratory failure) UNCONTROLLED ASTHMA


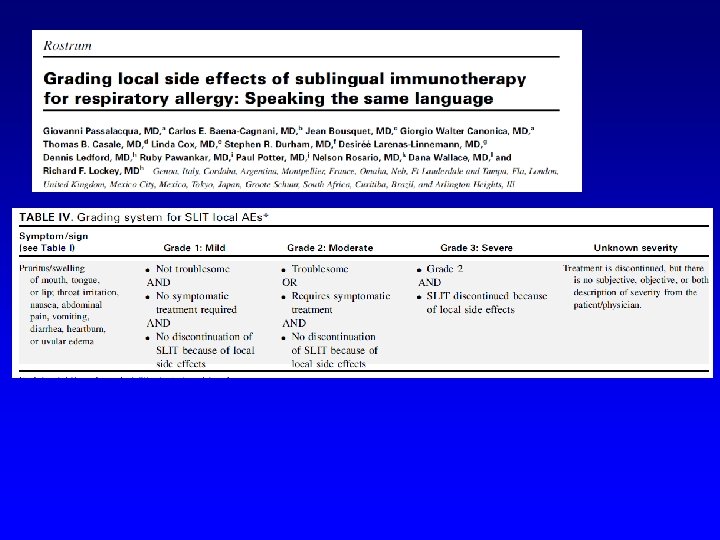
SLIT No fatal event reported since 1986 15 cases of anaphylaxis in 20 years

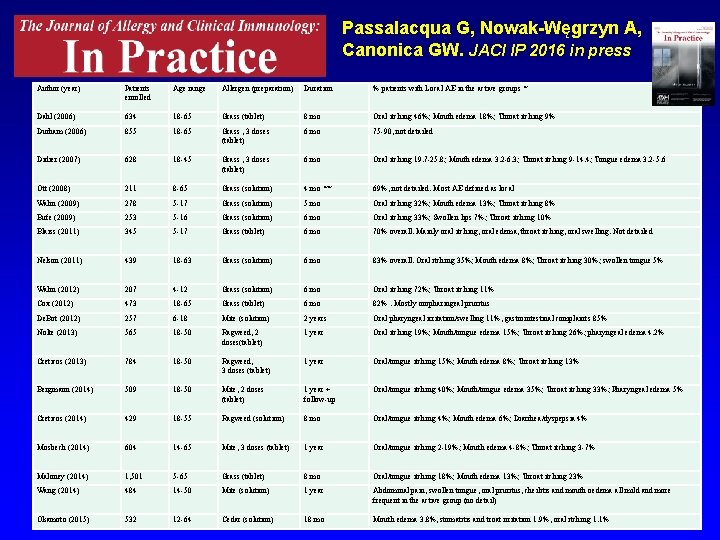
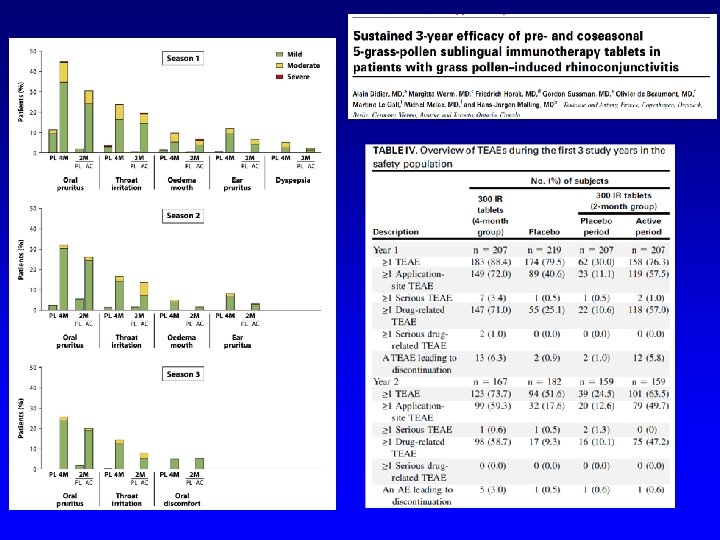
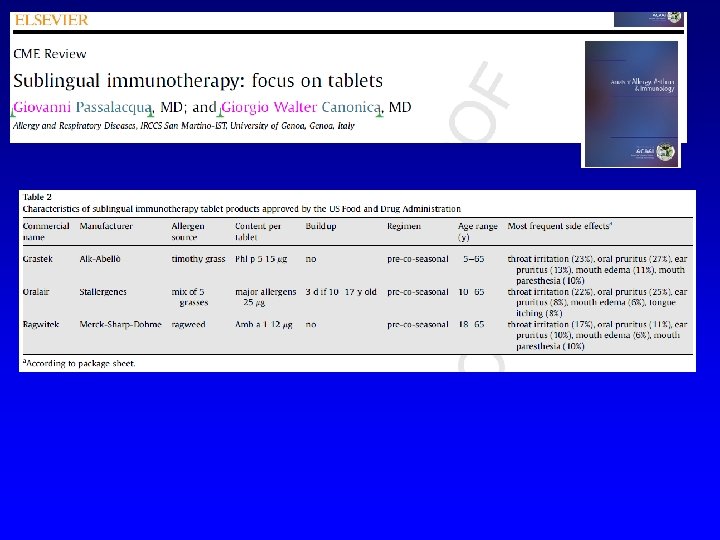
Passalacqua G, Nowak-Węgrzyn A, Canonica GW. JACI IP 2016 in press Author (year) Patients enrolled Age range Allergen (preparation) Duration % patients with Local AE in the active groups * Dahl (2006) 634 18 -65 Grass (tablet) 8 mo Oral itching 46%; Mouth edema 18%; Throat itching 9% Durham (2006) 855 18 -65 Grass , 3 doses (tablet) 6 mo 75 -90, not detailed Didier (2007) 628 18 -45 Grass , 3 doses (tablet) 6 mo Oral itching 19. 7 -25. 8; Mouth edema 3. 2 -6. 3; Throat itching 9 -14. 4; Tongue edema 3. 2 -5. 6 Ott (2008) 211 8 -65 Grass (solution) 4 mo ** 69%, not detailed. Most AE defined as local Wahn (2009) 278 5 -17 Grass (solution) 5 mo Oral itching 32%; Mouth edema 13%; Throat itching 8% Bufe (2009) 253 5 -16 Grass (solution) 6 mo Oral itching 33%; Swollen lips 7%; Throat itching 10% Blaiss (2011) 345 5 -17 Grass (tablet) 6 mo 70% overall. Mainly oral itching, oral edema, throat itching, oral swelling. Not detailed Nelson (2011) 439 18 -63 Grass (solution) 6 mo 83% overall. Oral itching 35%; Mouth edema 8%; Throat itching 30%; swollen tongue 5% Wahn (2012) 207 4 -12 Grass (solution) 6 mo Oral itching 72%; Throat itching 11% Cox (2012) 473 18 -65 Grass (tablet) 6 mo 82%. Mostly oropharingeal pruritus De. Bot (2012) 257 6 -18 Mite (solution) 2 years Oral pharyngeal irritation/swelling 11%, gastrointestinal complaints 85% Nolte (2013) 565 18 -50 Ragweed, 2 doses(tablet) 1 year Oral itching 19%; Mouth/tongue edema 15%; Throat itching 26%; pharyngeal edema 4. 2% Creticos (2013) 784 18 -50 Ragweed, 3 doses (tablet) 1 year Oral/tongue itching 15%; Mouth edema 8%; Throat itching 13% Bergmann (2014) 509 18 -50 Mite, 2 doses (tablet) 1 year + follow-up Oral/tongue itching 40%; Mouth/tongue edema 35%; Throat itching 33%; Pharyngeal edema 5% Creticos (2014) 429 18 -55 Ragweed (solution) 8 mo Oral/tongue itching 4%; Mouth edema 6%; Diarrhea/dyspepsia 4% Mosbech (2014) 604 14 -65 Mite, 3 doses (tablet) 1 year Oral/tongue itching 2 -19%; Mouth edema 4 -8%; Throat itching 3 -7% Maloney (2014) 1, 501 5 -65 Grass (tablet) 8 mo Oral/tongue itching 18%; Mouth edema 13%; Throat itching 23% Wang (2014) 484 14 -50 Mite (solution) 1 year Abdominal pain, swollen tongue, oral pruritus, cheilitis and mouth oedema all mild and more frequent in the active group (no detail) Okamoto (2015) 532 12 -64 Cedar (solution) 18 mo Mouth edema 3. 8%, stomatitis and troat irritation 1. 9%, oral itching 1. 1%




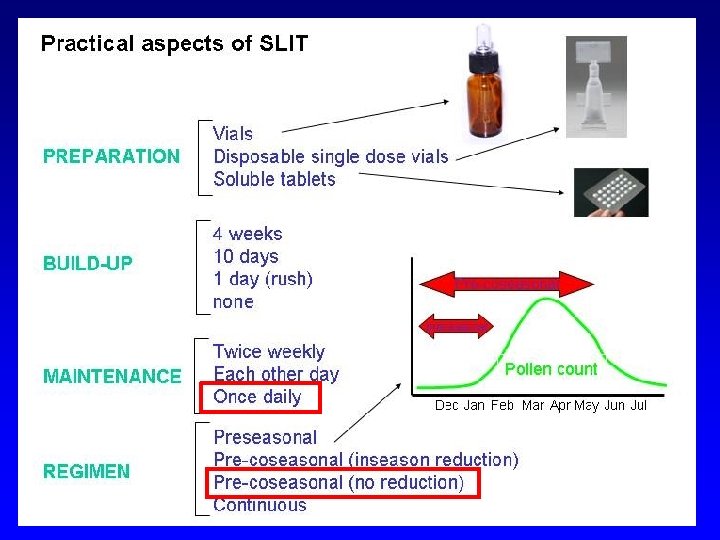
Aspetti pratici. In Italia è formalmente un “named patient product” (preparato dalla ditta per ciascun paziente dietro indicazione), anche se ad oggi i vaccini per AIT vengono preparati su scala industriale, come i farmaci e quindi uguali per tutti i pazienti. Due soli prodotti SLIT (graminacee) sono registrati come farmaco Gli estratti sono standardizzati (ossia è nota la quantità di allergene maggiore e la potenza) Con la SCIT Si effettua una fase di induzione graduale (solitamente 1/sett per 2 mesi), seguita da una fase di mantenimento (1/mese). Con la SLIT la fase di induzione può essere omessa Per allergeni pollinici si può effettuare un trattamento precostagionale. Per allergeni perenni, il trattamento è continuativo. Durata consigliata 3 -5 anni, da sospendere se dopo 2 anni non si ha beneficio.

SCIT: PRACTICAL ASPECTS • Ascertain that the dose and preparation are correct • Assess the clinical condition of the patient • Record date, hour, dose, reactions at previous injection • Use upper outer surface of arm • Ensure sterile technique • Use 1 m. L syringe • Inject at 45º by deep subcutaneous route • Record any local/systemic reaction • A waiting period of 30 min after injection is recommended

COSA OCCORRE PER LA SCIT: Adrenalina (iniezione i. m. ) Broncodilatatore short acting Steroide orale e i. v. Antistaminico orale e i. v. Set da infusione Ossigeno Ambu

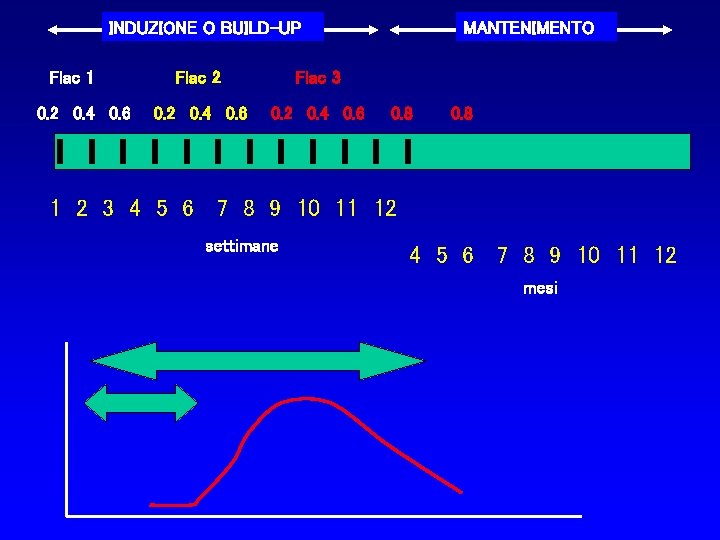
INDUZIONE O BUILD-UP Flac 1 0. 2 0. 4 0. 6 Flac 2 Flac 3 0. 2 0. 4 0. 6 MANTENIMENTO 0. 8 1 2 3 4 5 6 7 8 9 10 11 12 settimane 4 5 6 7 8 9 10 11 12 mesi

SLIT: PRACTICAL ASPECTS SLIT is self-administered at home by the patient. SLIT can be adminstered as mono-dose vials, drops, pre dosed spray or tablets After prescription, the first dose must be given under direct medical control The preparation (drops, tablets, spray-dose) should be assumed in the morning, being the patient fastened. The dose should be kept under the tongue for 1 -2 minutes (until dissolved for tablets), then swallowed. The patient must be instructed on the possible (local) side effects, and on how to manage them.


The omission of the build-up phase seems not to increase the risk of adverse events. Build up is usually not done with the more recent tablet preparations Short build-up courses (1 -5 days) can be applied, according to the manufacturer’s suggestion and to own experience

Explain to patients the possible side effects Explain that side effects tend to disappear after few doses Suggest medications (e. g. oral antihistamines) to control local side effects if any Administer the first dose under medical supervision

Practical aspects. SCIT BUILD-UP: Usually recommended (4 -8 wks) MAINTENANCE: usually every 4 wks DURATION: 3 -5 yrs PROTOCOL: continuous or pre-coseasonal (with the MPLadjuvanted SCIT, only 4 preseasonal injections) SLIT PREPARATION: drops, pre-dosed spray, tablets BUILD-UP: Very short (days) or absent MAINTEINANCE: Preferably daily (can vary according to the manufacturer). DURATION: 3 -5 yrs PROTOCOL: Preferred pre- coseasonal (continuous for HDM)

INIZIO: Prima della stagione di pollinazione (2 mesi) In qualsiasi momento per i perenni SCHEMA: Tradizionale, cluster, rush MANTENIMENTO: Prestagionale, precostagionale, continuo DURATA: Almeno 3 -5 anni, poi se beneficio sospendere Se non beneficio dopo 2 anni sospendere VALUTAZIONE: Clinica (riduzione dei sintomi e dei farmaci)



PREMEDICATION: PROS: Preventing reactions Avoiding severe reactions Diminishing reactions’intensity ? CONS: May mask symptoms’ onset May delay appropriate treatment


JACI 2016

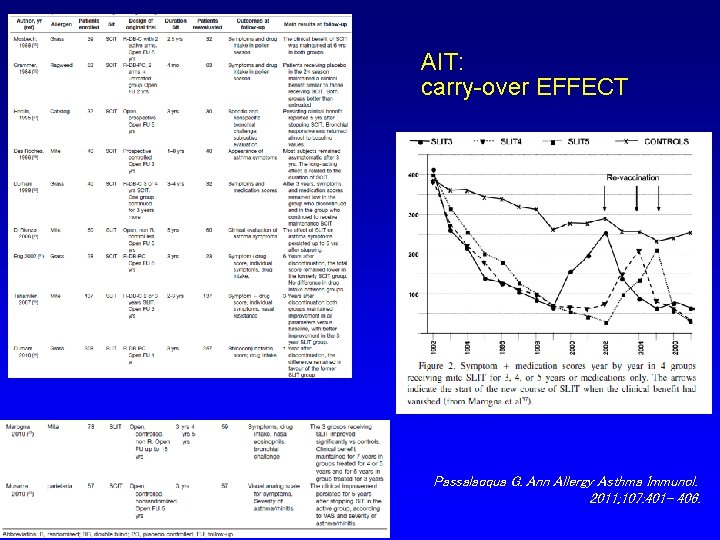
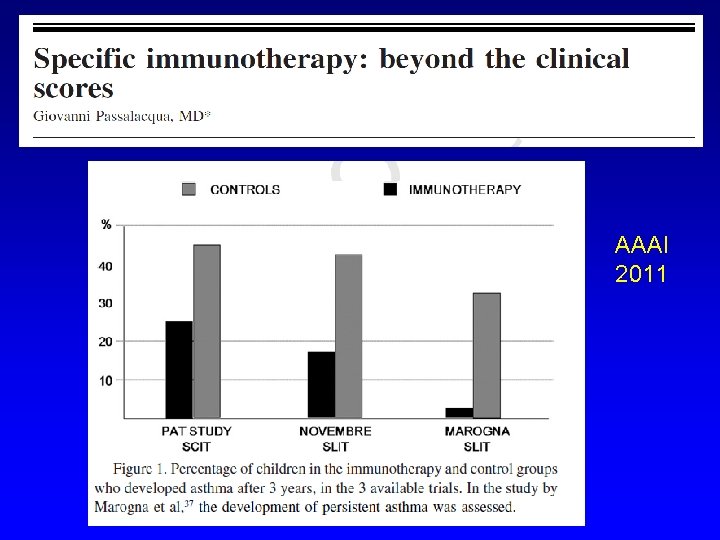
EFFETTI “SPECIALI” DELL’AIT Efficacia a lungo termine dopo la sospensione Prevenzione di nuove sensibilizzazioni Riduzione del rischio di insorgenza di asma Modificazione della risposta immunitaria

AIT: carry-over EFFECT Passalacqua G. Ann Allergy Asthma Immunol. 2011; 107: 401– 406.


AAAI 2011


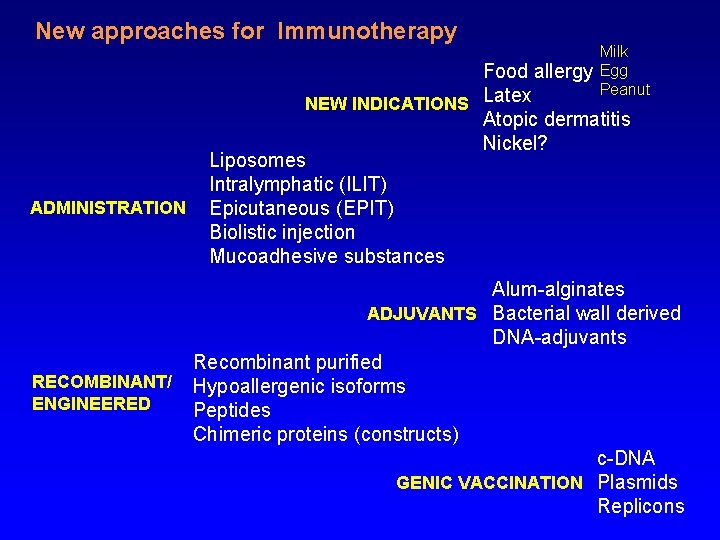
New approaches for Immunotherapy ADMINISTRATION RECOMBINANT/ ENGINEERED Milk Egg Peanut Food allergy NEW INDICATIONS Latex Atopic dermatitis Nickel? Liposomes Intralymphatic (ILIT) Epicutaneous (EPIT) Biolistic injection Mucoadhesive substances Alum-alginates ADJUVANTS Bacterial wall derived DNA-adjuvants Recombinant purified Hypoallergenic isoforms Peptides Chimeric proteins (constructs) c-DNA GENIC VACCINATION Plasmids Replicons

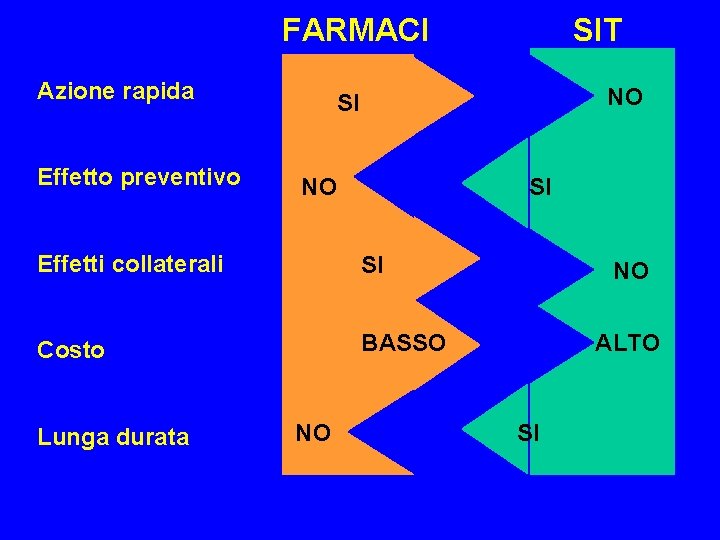
FARMACI Azione rapida Effetto preventivo NO SI Effetti collaterali SI Costo BASSO Lunga durata SIT NO NO ALTO SI

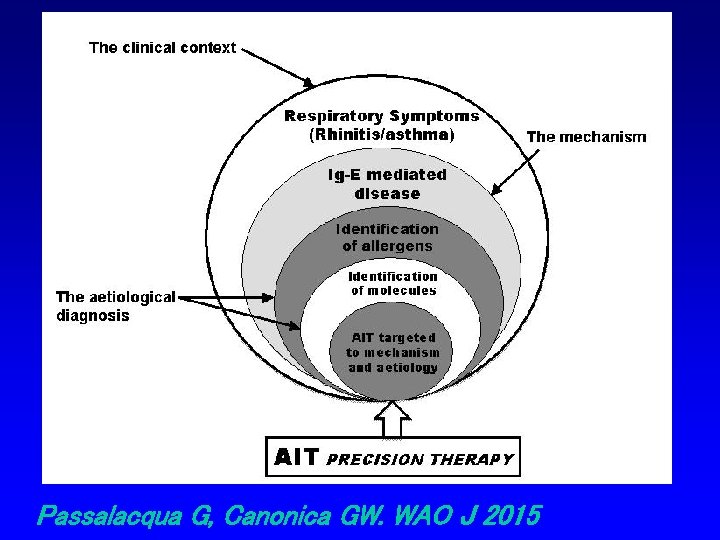
Passalacqua G, Canonica GW. WAO J 2015

CONCLUSIONI Farmacoterapia e immunoterapia hanno meccanismi diversi Il loro effetto è additivo L’ITS consente un risparmio di farmaci sintomatici L’ITS ha effetti preventivi e a lungo termine che i farmaci non hanno L’ITS agisce contemporaneamente su naso e bronchi FARMACI E ITS NON SONO MUTUAMENTE ESCLUSIVI