Limiting Reagent and Percent Yield Created by Miss

Limiting Reagent and Percent Yield Created by: Miss K. Marshall

Limiting and Excess Reagents § Chemical reactions require a particular amount of reactants. § If there is not enough of a particular reactant, the amount of product formed will be limited. § Coefficients in balanced equations give the ratio of representative particles and the mole ratio

Limiting and Excess Reagents §

Limiting and Excess Reagents § Limiting reagent § the reagent that determines the amount of product that can be formed by a reaction § A reaction will only occur until all of the limiting reagent is used up § Excess reagent § The reactant that is not completely used up in a reaction

Limiting and Excess Reagents § How do we solve these problems? § Convert to moles 1 st. § Identify limiting reagent § Make sure your answer makes sense

Determining the Limiting Reagent in a Reaction §

Using a Limiting Reagent to Find the Quantity of a Product §

Percent Yield §
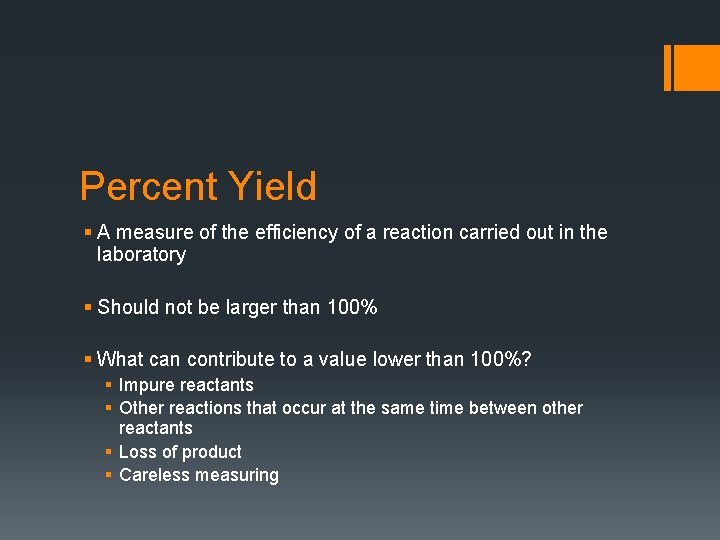
Percent Yield § A measure of the efficiency of a reaction carried out in the laboratory § Should not be larger than 100% § What can contribute to a value lower than 100%? § Impure reactants § Other reactions that occur at the same time between other reactants § Loss of product § Careless measuring
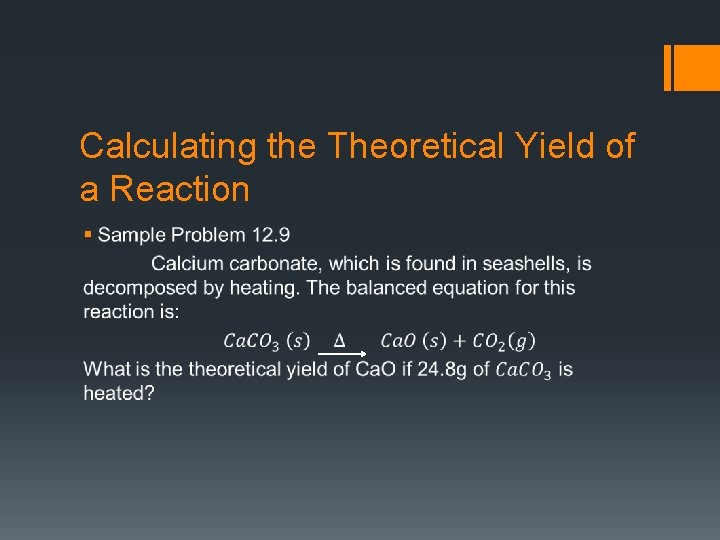
Calculating the Theoretical Yield of a Reaction §

Calculating the Percent Yield of a Reaction §
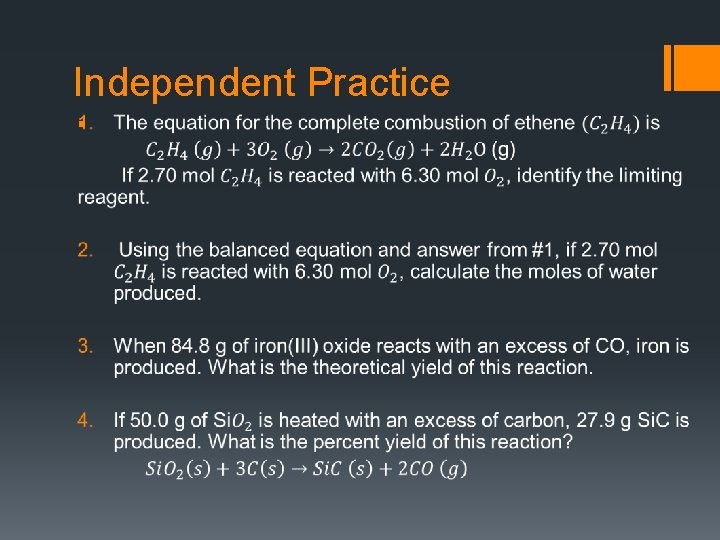
Independent Practice §

More Independent Practice §
- Slides: 13