Limiting Reactant STOICHIOMETRY BACK TO OUR CAKE ANALOGY

Limiting Reactant STOICHIOMETRY

BACK TO OUR CAKE ANALOGY � 1 cake mix + 3 eggs + 1 cup water 1 cake � If you have 2 mixes, 3 eggs and 5 cups of water, how many cakes can you make? � In this case we would call the eggs the limiting reactant. We can only make one cake because we don’t have enough eggs to match the other materials that are available. In this case we would make 1 cake and have 1 mix and 4 cups water left over.
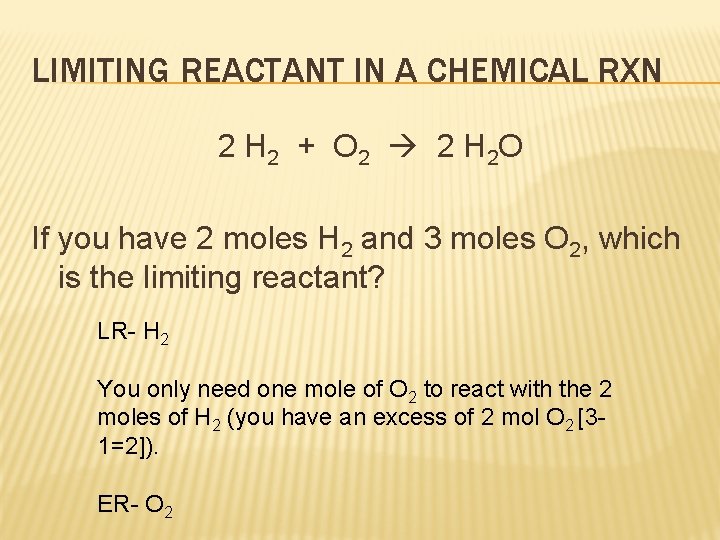
LIMITING REACTANT IN A CHEMICAL RXN 2 H 2 + O 2 2 H 2 O If you have 2 moles H 2 and 3 moles O 2, which is the limiting reactant? LR- H 2 You only need one mole of O 2 to react with the 2 moles of H 2 (you have an excess of 2 mol O 2 [31=2]). ER- O 2
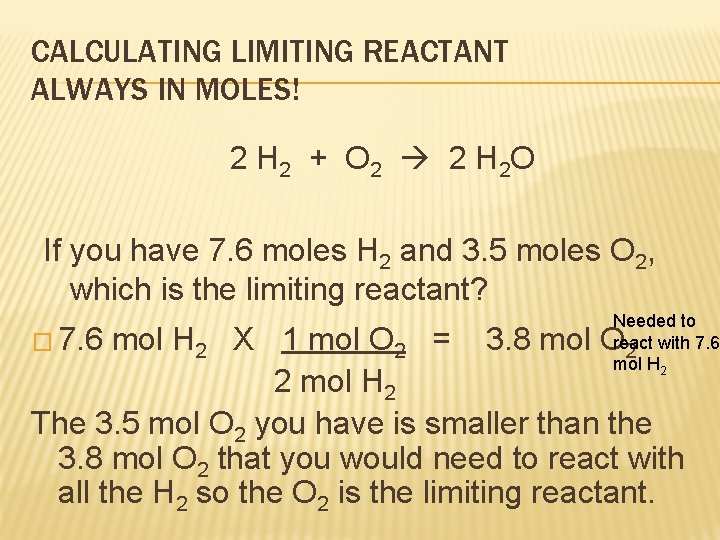
CALCULATING LIMITING REACTANT ALWAYS IN MOLES! 2 H 2 + O 2 2 H 2 O If you have 7. 6 moles H 2 and 3. 5 moles O 2, which is the limiting reactant? � 7. 6 Needed to react 2 with 7. 6 mol H 2 X 1 mol O 2 = 3. 8 mol O 2 mol H 2 The 3. 5 mol O 2 you have is smaller than the 3. 8 mol O 2 that you would need to react with all the H 2 so the O 2 is the limiting reactant.

� Remember to balance the equation first!
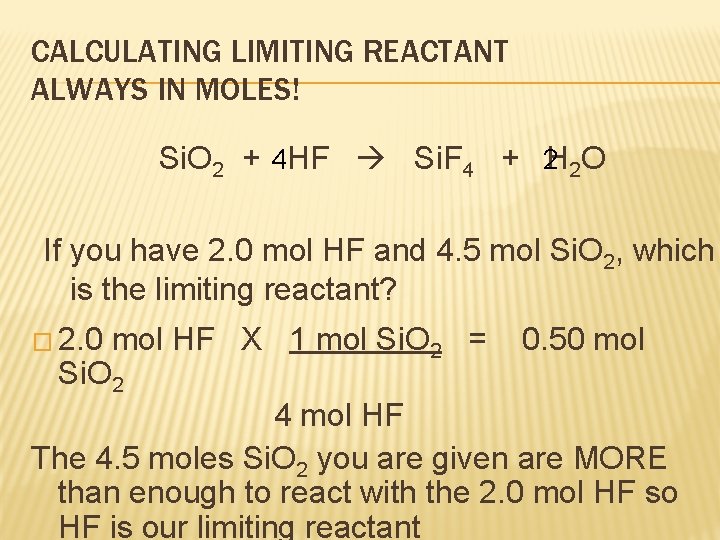
CALCULATING LIMITING REACTANT ALWAYS IN MOLES! Si. O 2 + 4 HF Si. F 4 + 2 H 2 O If you have 2. 0 mol HF and 4. 5 mol Si. O 2, which is the limiting reactant? � 2. 0 mol HF X 1 mol Si. O 2 = Si. O 2 0. 50 mol 4 mol HF The 4. 5 moles Si. O 2 you are given are MORE than enough to react with the 2. 0 mol HF so HF is our limiting reactant
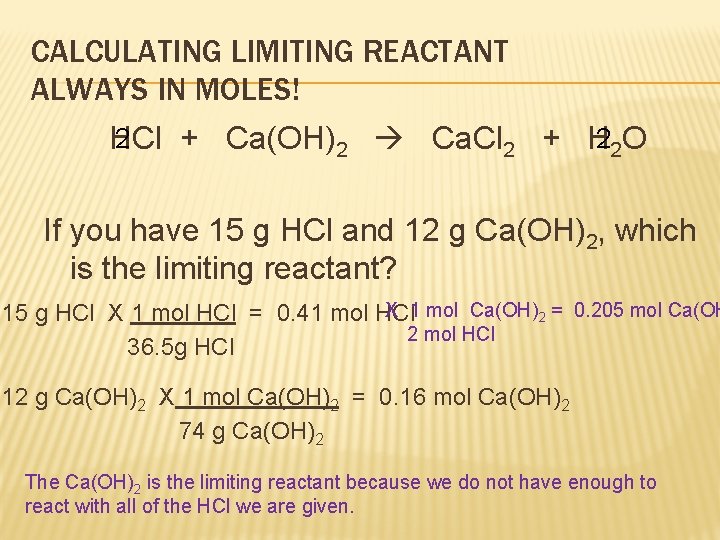
CALCULATING LIMITING REACTANT ALWAYS IN MOLES! 2 HCl + Ca(OH)2 Ca. Cl 2 + H 22 O If you have 15 g HCl and 12 g Ca(OH)2, which is the limiting reactant? X 1 mol Ca(OH)2 = 0. 205 mol Ca(OH 15 g HCl X 1 mol HCl = 0. 41 mol HCl 2 mol HCl 36. 5 g HCl 12 g Ca(OH)2 X 1 mol Ca(OH)2 = 0. 16 mol Ca(OH)2 74 g Ca(OH)2 The Ca(OH)2 is the limiting reactant because we do not have enough to react with all of the HCl we are given.
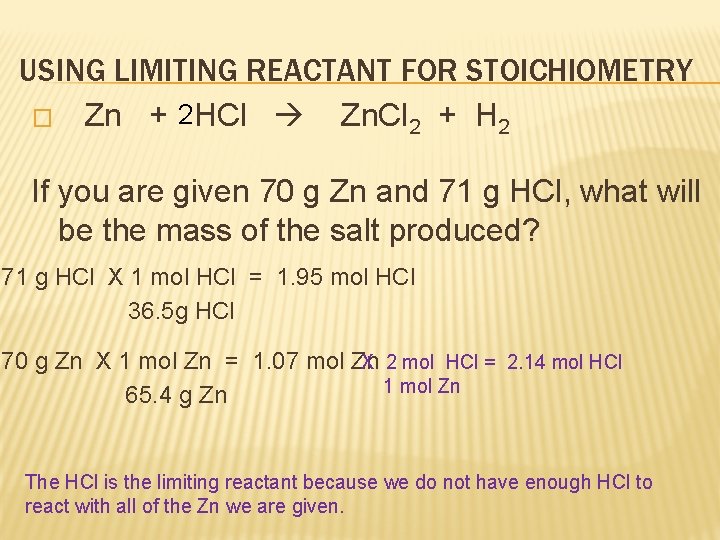
USING LIMITING REACTANT FOR STOICHIOMETRY � Zn + 2 HCl Zn. Cl 2 + H 2 If you are given 70 g Zn and 71 g HCl, what will be the mass of the salt produced? 71 g HCl X 1 mol HCl = 1. 95 mol HCl 36. 5 g HCl 70 g Zn X 1 mol Zn = 1. 07 mol Zn X 2 mol HCl = 2. 14 mol HCl 1 mol Zn 65. 4 g Zn The HCl is the limiting reactant because we do not have enough HCl to react with all of the Zn we are given.
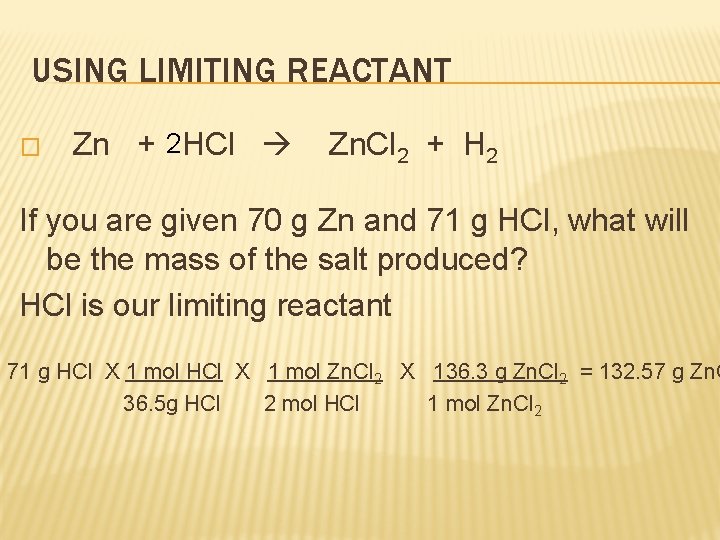
USING LIMITING REACTANT � Zn + 2 HCl Zn. Cl 2 + H 2 If you are given 70 g Zn and 71 g HCl, what will be the mass of the salt produced? HCl is our limiting reactant 71 g HCl X 1 mol Zn. Cl 2 X 136. 3 g Zn. Cl 2 = 132. 57 g Zn. C 36. 5 g HCl 2 mol HCl 1 mol Zn. Cl 2

% YIELD � If you did the reaction on the last two slides and you only collected 110 g of Zn. Cl 2, what is your % yield? % Yield = mass collected mass expected 110 g X 132. 57 g X 100% = 82. 97%

% YIELD �% yield = experimental yield x 100% theoretical yield % expressing what you u collected vs what you were supposed to collect.
- Slides: 11