Limiting Excess Reactants How do you know which





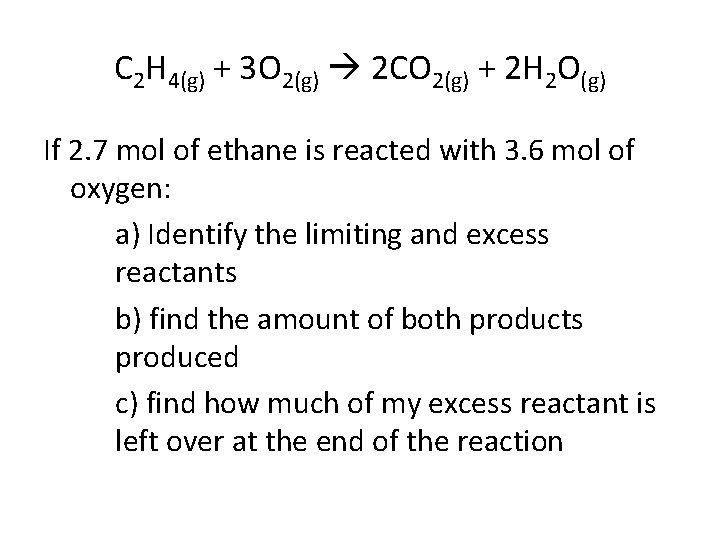
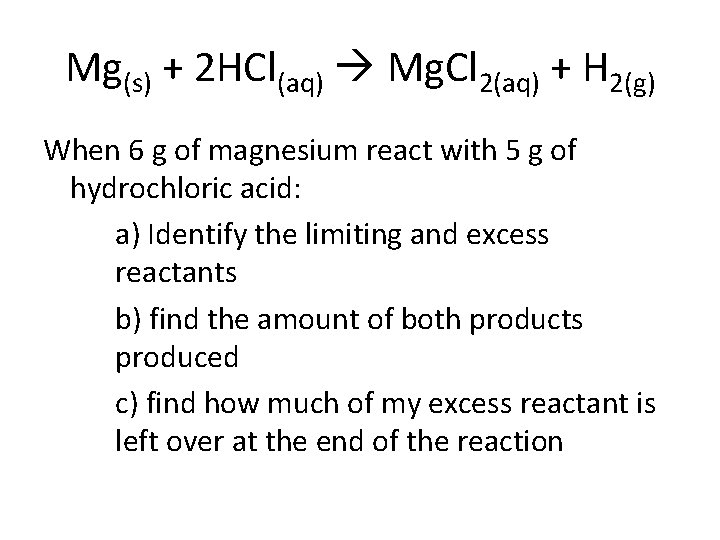

- Slides: 12

Limiting & Excess Reactants How do you know which one is which?

What does limiting & excess mean? • Limiting Reactant - the reactant that runs out first in a chemical reaction, thus determining the amount of product produced • Excess Reactant - the reactant that there is a quantity of left over after a chemical reaction • *The excess reactant should be the cheaper reactant since we do not like to waste unused chemical • *The excess reactant should be easy to isolate from the product(s) once the reaction is complete since we do not want contamination

Identifying Limiting and Excess Reagents • WHY DO WE CARE? ? • It is often desirable to know how much excess reagent is required to ensure that a reaction goes to completion. – Plus, when you run out of your limiting reactant, you wont make any more product! • When you know the quantity of more than one reagent, you may need to know which one will limit the reaction.

Steps: 1. Convert all quantities to moles 2. Compare via mole ratio 3. Look back at original quantities

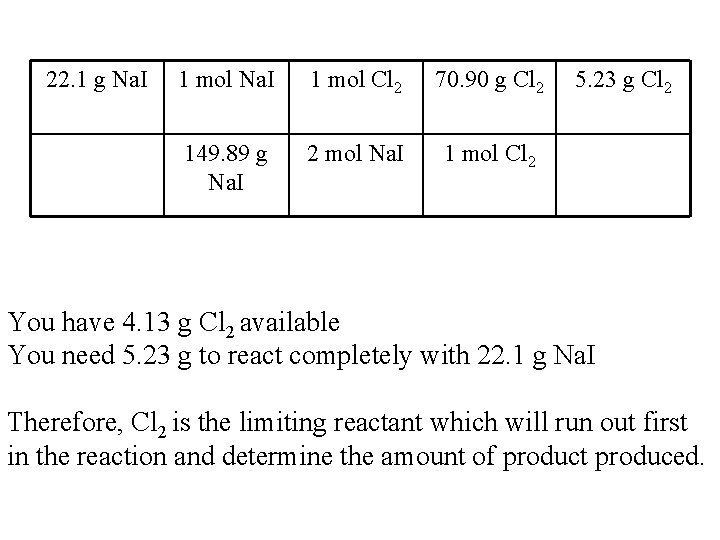
Practice Problem 2 Na. I + Cl 2 2 Na. Cl + I 2 • 1. You are given 22. 1 g of Na. I and 4. 13 g of Cl 2. What is the limiting reactant? • Pick one of the values given for your reactants and through stoichiometry find out how much you need of the other reactant.

22. 1 g Na. I 1 mol Cl 2 70. 90 g Cl 2 149. 89 g Na. I 2 mol Na. I 1 mol Cl 2 5. 23 g Cl 2 You have 4. 13 g Cl 2 available You need 5. 23 g to react completely with 22. 1 g Na. I Therefore, Cl 2 is the limiting reactant which will run out first in the reaction and determine the amount of product produced.

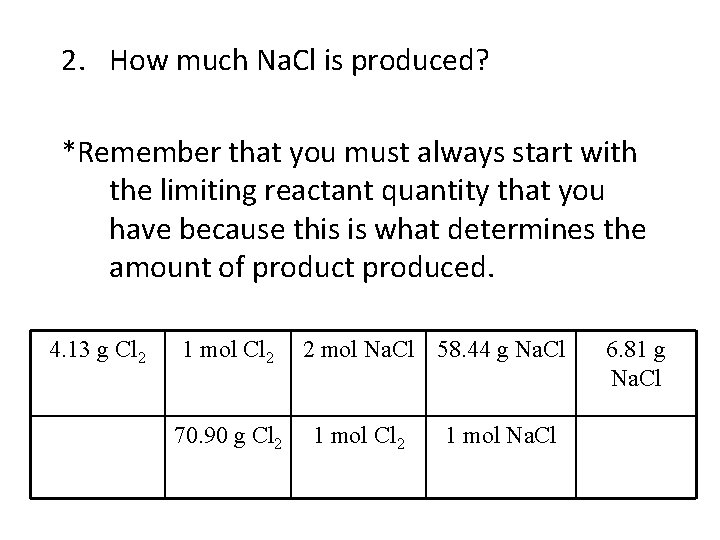
2. How much Na. Cl is produced? *Remember that you must always start with the limiting reactant quantity that you have because this is what determines the amount of product produced. 4. 13 g Cl 2 1 mol Cl 2 70. 90 g Cl 2 2 mol Na. Cl 58. 44 g Na. Cl 1 mol Cl 2 1 mol Na. Cl 6. 81 g Na. Cl

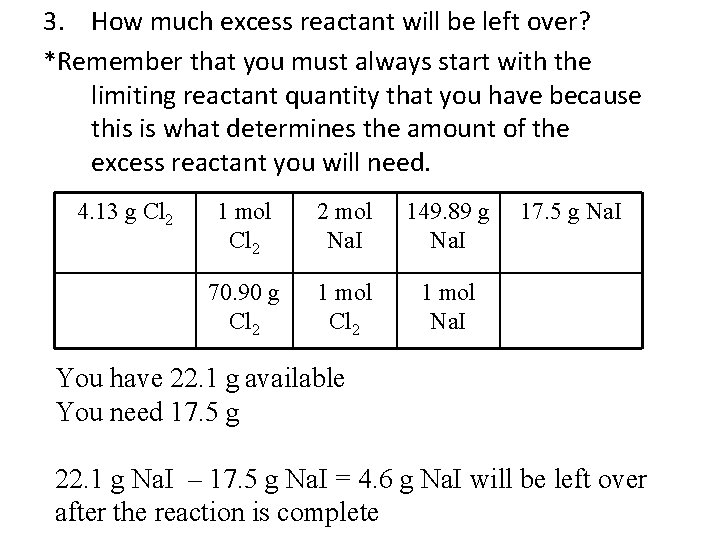
3. How much excess reactant will be left over? *Remember that you must always start with the limiting reactant quantity that you have because this is what determines the amount of the excess reactant you will need. 4. 13 g Cl 2 1 mol Cl 2 2 mol Na. I 149. 89 g Na. I 70. 90 g Cl 2 1 mol Na. I 17. 5 g Na. I You have 22. 1 g available You need 17. 5 g 22. 1 g Na. I – 17. 5 g Na. I = 4. 6 g Na. I will be left over after the reaction is complete
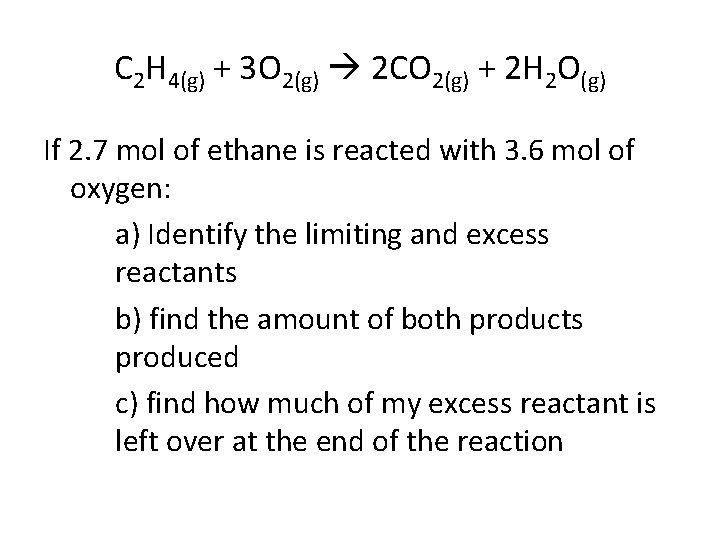
C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O(g) If 2. 7 mol of ethane is reacted with 3. 6 mol of oxygen: a) Identify the limiting and excess reactants b) find the amount of both products produced c) find how much of my excess reactant is left over at the end of the reaction
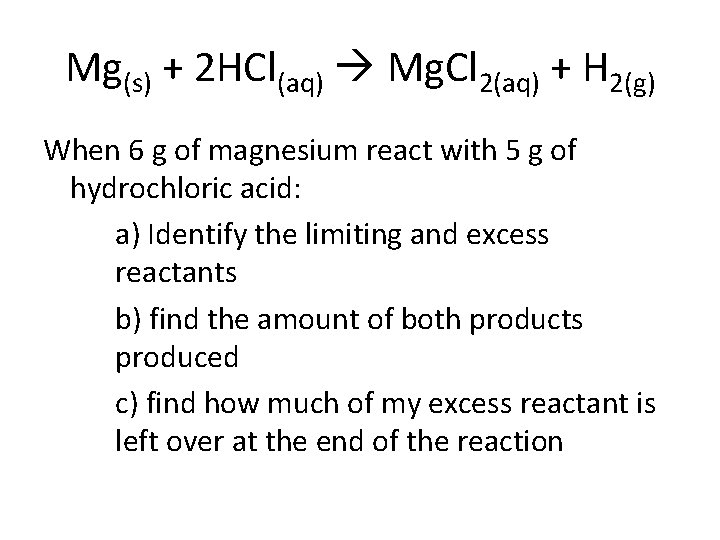
Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) When 6 g of magnesium react with 5 g of hydrochloric acid: a) Identify the limiting and excess reactants b) find the amount of both products produced c) find how much of my excess reactant is left over at the end of the reaction

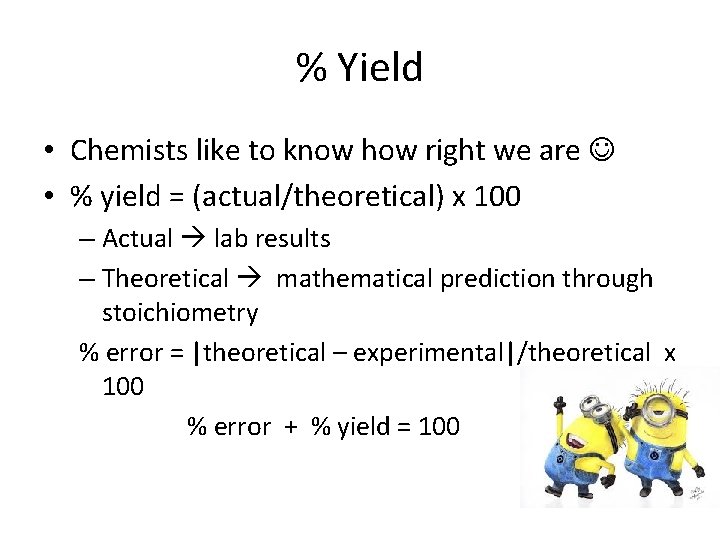
% Yield • Chemists like to know how right we are • % yield = (actual/theoretical) x 100 – Actual lab results – Theoretical mathematical prediction through stoichiometry % error = |theoretical – experimental|/theoretical x 100 % error + % yield = 100

Independent Practice