Limiting Excess Cookie Stoichiometry To make one batch

Limiting & Excess

Cookie Stoichiometry • To make one batch, it • How many batches could you requires: make with… • 2 eggs • 2 cups of flour • 2/3 cups of butter – 4 eggs – 6 cups of flour – 2/3 cups of butter – The cups of butter was your limiting reagent – The eggs and flour were your excess reagents – But how could you show this mathematically…?

2 Together…. 3 on your Own! Whiteboard!

Example 1 20. 00 g of H 2 react with 100. 0 g of O 2 according to the reaction 2 H 2 + O 2 2 H 2 O Which reactant is limiting and which reactant is in excess?

Example 1 1. Calculate the number of moles of a product formed. Mole of H 2 O (based on H 2) Mole of H 2 O (based on O 2) = 20. 00 g H 2 x = 100. 0 g O 2 x 1 mol H 2 2. 0 g H 2 1 mol O 2 32. 0 g O 2 x x 2 mol H 2 O 1 mol O 2 10. 0 mole H 2 O = = 6. 25 mol H 2 O

Example 1 Determine the limiting and excess reactants. O 2 is the limiting reactant. H 2 is the excess reactant. 2. How much H 2 (in grams) is left after O 2 runs out?
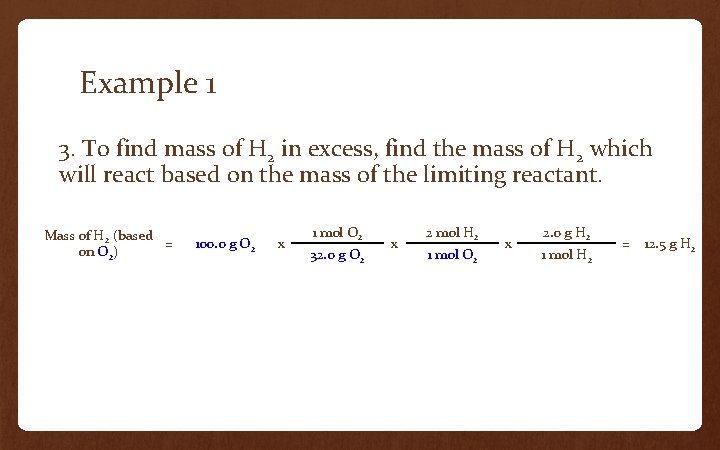
Example 1 3. To find mass of H 2 in excess, find the mass of H 2 which will react based on the mass of the limiting reactant. Mass of H 2 (based = on O 2) 100. 0 g O 2 x 1 mol O 2 32. 0 g O 2 x 2 mol H 2 1 mol O 2 x 2. 0 g H 2 1 mol H 2 = 12. 5 g H 2
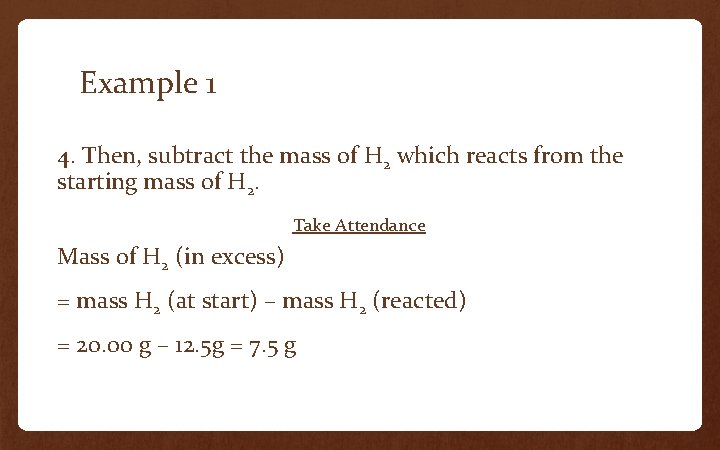
Example 1 4. Then, subtract the mass of H 2 which reacts from the starting mass of H 2. Take Attendance Mass of H 2 (in excess) = mass H 2 (at start) – mass H 2 (reacted) = 20. 00 g – 12. 5 g = 7. 5 g

Example 2 If 56. 8 g of Fe. Cl 2, 14. 9 g of KNO 3 and 40. 0 g of HCl are mixed according to the reaction Fe. Cl 2 + KNO 3 + HCl Fe. Cl 3 + NO + H 2 O + KCl a) What is the limiting reactant? b) How many grams of each “excess” reactant left in the reaction vessel?

Example 2 If 56. 8 g of Fe. Cl 2, 14. 9 g of KNO 3 and 40. 0 g of HCl are mixed according to the reaction 3 Fe. Cl 2 + KNO 3 + 4 HCl 3 Fe. Cl 3 + NO + 2 H 2 O + KCl a) What is the limiting reactant? b) How many grams of each “excess” reactant left in the reaction vessel?
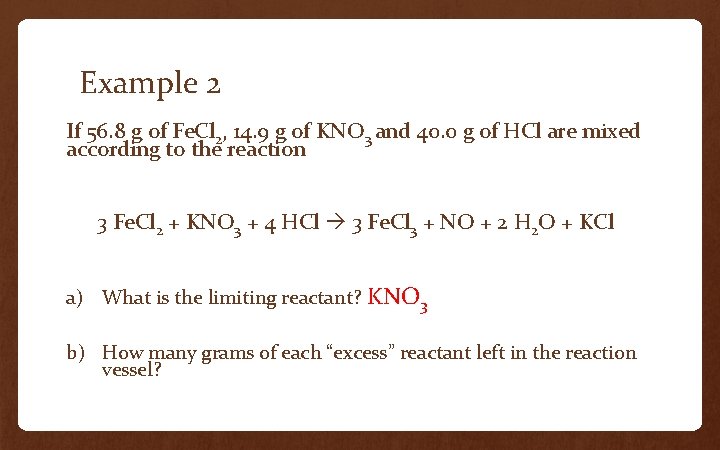
Example 2 If 56. 8 g of Fe. Cl 2, 14. 9 g of KNO 3 and 40. 0 g of HCl are mixed according to the reaction 3 Fe. Cl 2 + KNO 3 + 4 HCl 3 Fe. Cl 3 + NO + 2 H 2 O + KCl a) What is the limiting reactant? KNO 3 b) How many grams of each “excess” reactant left in the reaction vessel?
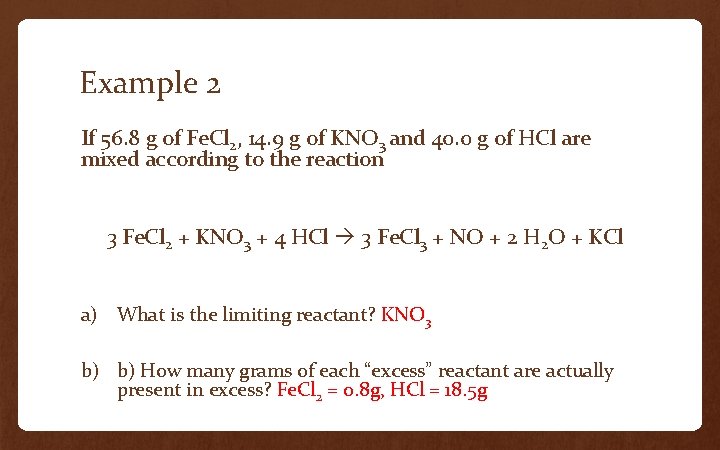
Example 2 If 56. 8 g of Fe. Cl 2, 14. 9 g of KNO 3 and 40. 0 g of HCl are mixed according to the reaction 3 Fe. Cl 2 + KNO 3 + 4 HCl 3 Fe. Cl 3 + NO + 2 H 2 O + KCl a) What is the limiting reactant? KNO 3 b) b) How many grams of each “excess” reactant are actually present in excess? Fe. Cl 2 = 0. 8 g, HCl = 18. 5 g


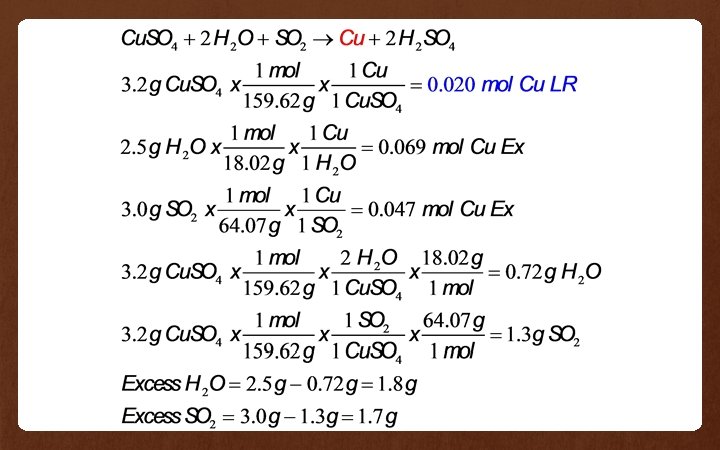
Example 3 If 3. 2 g of Cu. SO 4, 2. 5 g of water and 3. 0 g of SO 2 are reacted together in the reaction: Cu. SO 4 + H 2 O + SO 2 Cu + H 2 SO 4 • Which reactant is the limiting reactant? • What is the mass of each of the excess reactants?

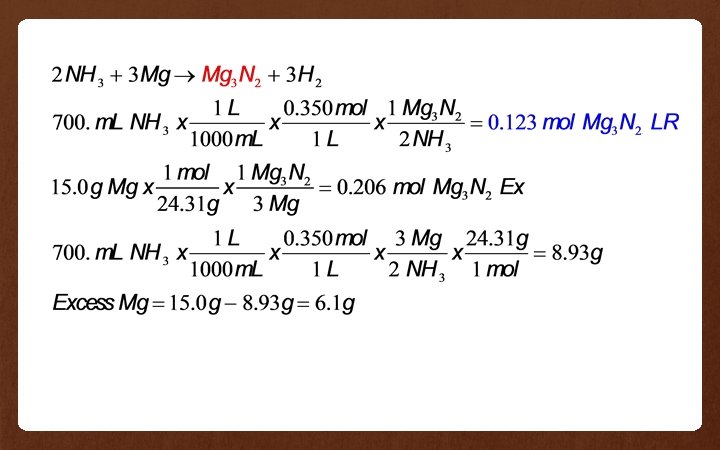
Example 4 700. m. L of 0. 350 M ammonia (NH 3) solution is mixed with 15. 0 g of solid magnesium to produce magnesium nitride and hydrogen gas. • Which reactant is in excess? • How much (in grams) of the excess reactant remains after the reaction is complete?

Example 5: For the reaction: Ti. O 2(aq) + B 4 C(g) + C(s) Ti. B 2(aq) + CO(g) If 11. 5 L of 0. 800 M Ti. O 2, 455 g of solid carbon and 184 L of B 4 C gas at STP react together. Find the amount (based on the original units given) of the reactants left over.
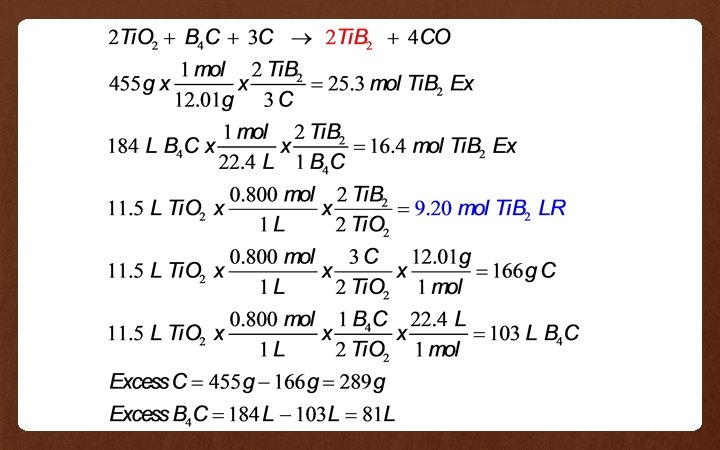
Example 5: For the reaction: 2 Ti. O 2(aq) + B 4 C(g) + 3 C(s) 2 Ti. B 2(aq) + 4 CO(g) If 11. 5 L of 0. 800 M Ti. O 2, 455 g of solid carbon and 184 L of B 4 C gas at STP react together. Find the amount (based on the original units given) of the reactants left over.
- Slides: 20