Light Quantitized Energy Quantum Theory EQ What does













- Slides: 26

Light, Quantitized Energy & Quantum Theory EQ: What does the Modern Atom look like? CVHS Chemistry Ch 5. 1 & 5. 2

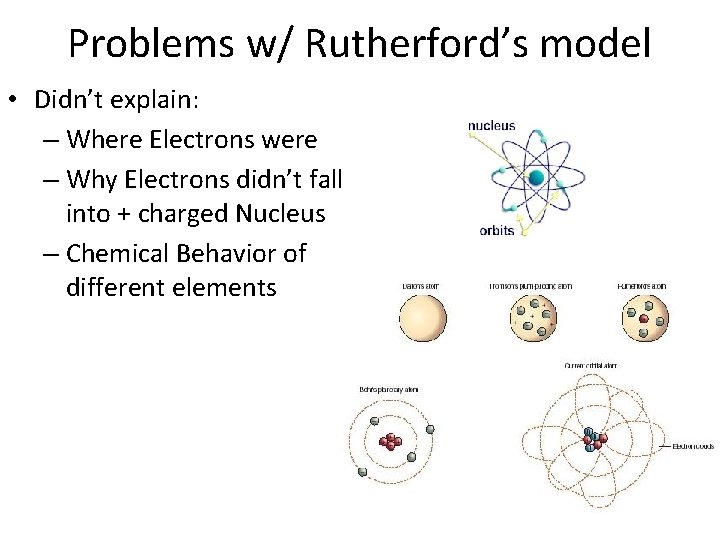
Problems w/ Rutherford’s model • Didn’t explain: – Where Electrons were – Why Electrons didn’t fall into + charged Nucleus – Chemical Behavior of different elements

Flame Tests

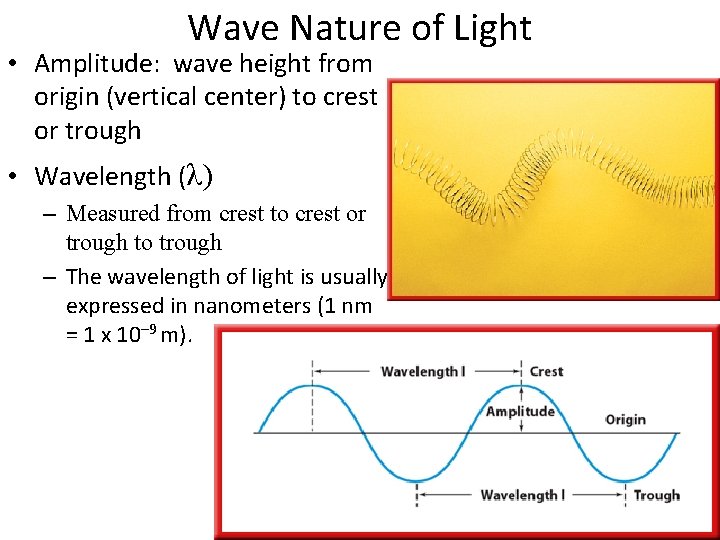
Wave Nature of Light • Amplitude: wave height from origin (vertical center) to crest or trough • Wavelength (λ) – Measured from crest to crest or trough to trough – The wavelength of light is usually expressed in nanometers (1 nm = 1 x 10– 9 m).

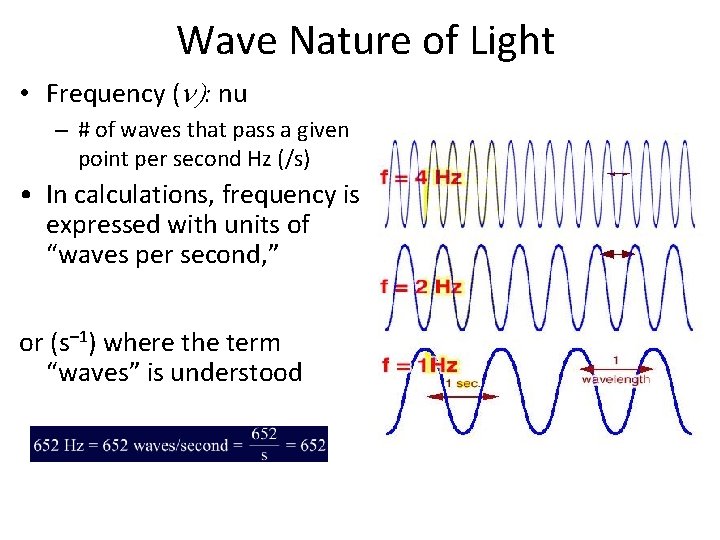
Wave Nature of Light • Frequency (n): nu – # of waves that pass a given point per second Hz (/s) • In calculations, frequency is expressed with units of “waves per second, ” or (s– 1) where the term “waves” is understood

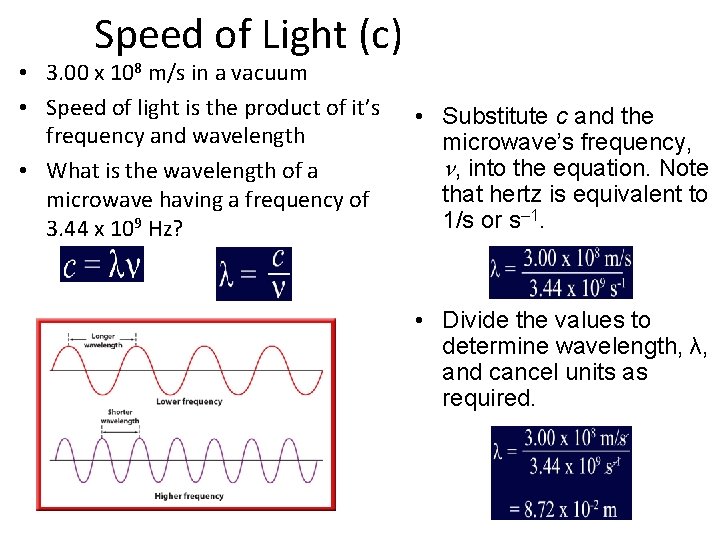
Speed of Light (c) • 3. 00 x 108 m/s in a vacuum • Speed of light is the product of it’s frequency and wavelength • What is the wavelength of a microwave having a frequency of 3. 44 x 109 Hz? • Substitute c and the microwave’s frequency, n, into the equation. Note that hertz is equivalent to 1/s or s– 1. • Divide the values to determine wavelength, λ, and cancel units as required.

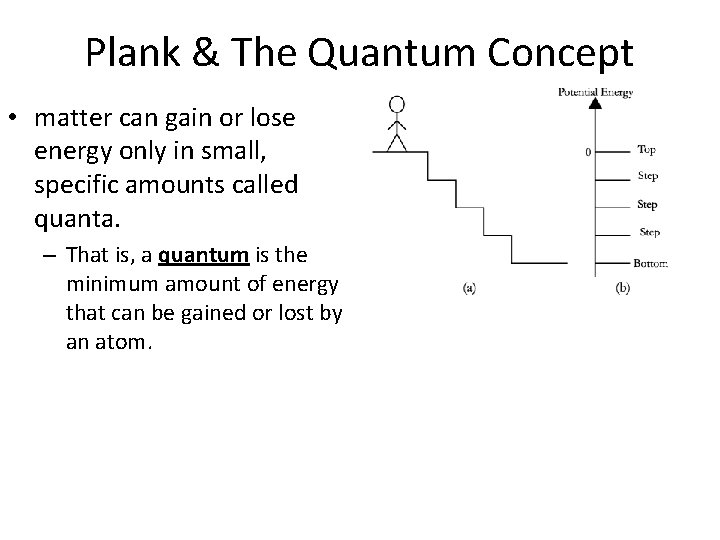
Plank & The Quantum Concept • matter can gain or lose energy only in small, specific amounts called quanta. – That is, a quantum is the minimum amount of energy that can be gained or lost by an atom.

Photoelectric Effect • In the photoelectric effect, electrons, called photoelectrons, are emitted from a metal’s surface when light of a certain frequency shines on the surface. • Further, Einstein proposed The photon of light must have enough energy to bump the electron off the metal

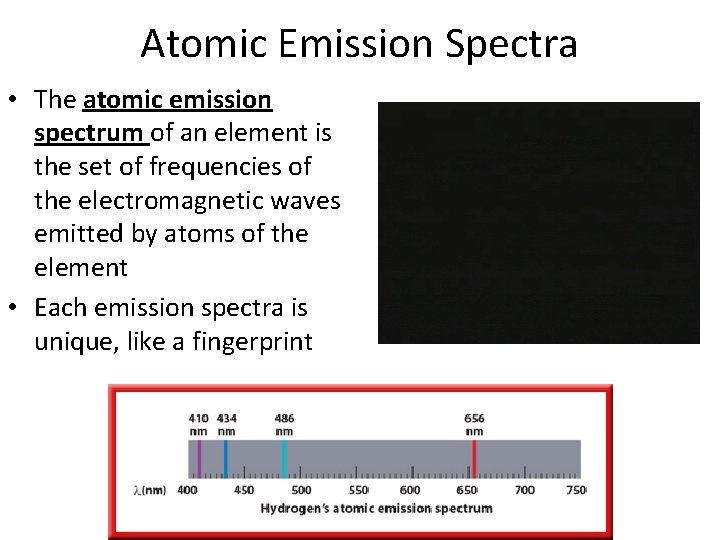
Atomic Emission Spectra • The atomic emission spectrum of an element is the set of frequencies of the electromagnetic waves emitted by atoms of the element • Each emission spectra is unique, like a fingerprint

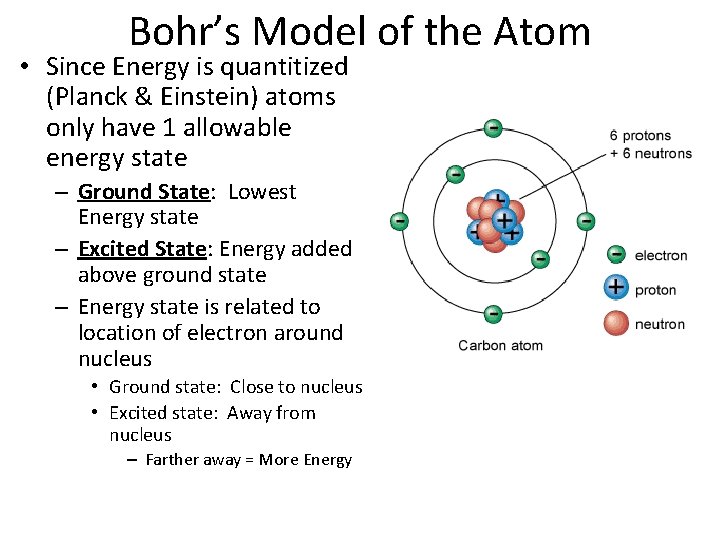
Bohr’s Model of the Atom • Since Energy is quantitized (Planck & Einstein) atoms only have 1 allowable energy state – Ground State: Lowest Energy state – Excited State: Energy added above ground state – Energy state is related to location of electron around nucleus • Ground state: Close to nucleus • Excited state: Away from nucleus – Farther away = More Energy

Louis de Broglie’s Atom • Treated electrons orbit as a wave NOTICE: Waves have mass!

Heisenberg Uncertainty Principle • Measuring the location or velocity of an electron changes the location and velocity of the electron • The Heisenberg uncertainty principle Can’t know velocity & position @ same time

Physicist Erwin Schrödinger (1926) & Wave Equations • Treated the electron as a wave – Aka: quantum mechanical model • Seemed to apply to all atoms, not just hydrogen like Bohr’s model • Puts electrons in distinct energy levels but doesn’t describe its path around the nucleus • Atomic Orbital: 3 -D region that describes an electrons most probable location around the nucleus – Sort of a fuzzy cloud – The circle represents the 90% probability area for finding an electron

Quantum Numbers • Principal Quantum Number (n) – Distance from the nucleus – The # of sublevels found in an energy level is equal to the number of the energy level • Energy Sublevels: describe by the shape of the atom’s orbitals – – s, spherical (1) p, peanut or dumbell (3) d, daisy (variable) (5) f, flower (variable) (7)

Energy Levels & Sublevels • Electrons w/in the same energy level but in different sublevels have similar amounts of energy • Sublevels – s, p, d, f – s has the lowest energy & f has the highest energy – Each energy level has the # of sublevels equal to the number of the energy level • Energy level 1 has – 1 s • Energy level 2 has – 2 s & 2 p • Energy level 3 has – 3 s & 3 p & 3 d Look at the shape of each orbital w/in a sublevel. Doesn’t it make sense that it takes more energy for an electron to move in an f pattern than an s pattern?

Electron Configurations • A specific # of electrons can fit into each sublevel • s – Holds 2 electrons • p – Holds 6 electrons • d – Holds 10 electrons • f – Holds 14 electrons

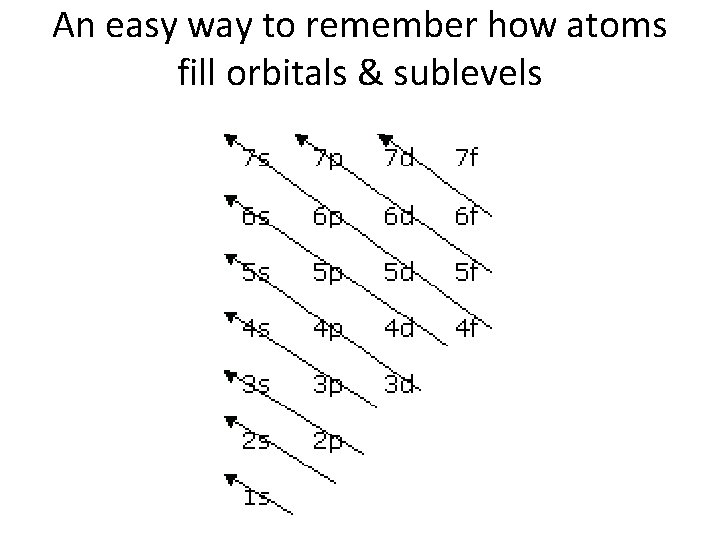
Rules for Electrons • Aufbau Principle: Electrons fill the lowest energy levels first • Hund’s Rule: At any sublevel, electrons go into orbitals one at a time until they have no choice but to pair. • Pauli Exclusion Principle: Any 2 e- in the same orbital must spin in opposite directions.

Electron Configurations • Electrons fill energy levels and sublevels in an orderly fashion w/ the lowest energy orbitals being filled first – This creates the most stable electron configuration • The shape of the modern periodic table is a direct result of the arrangement of electrons in the atom.

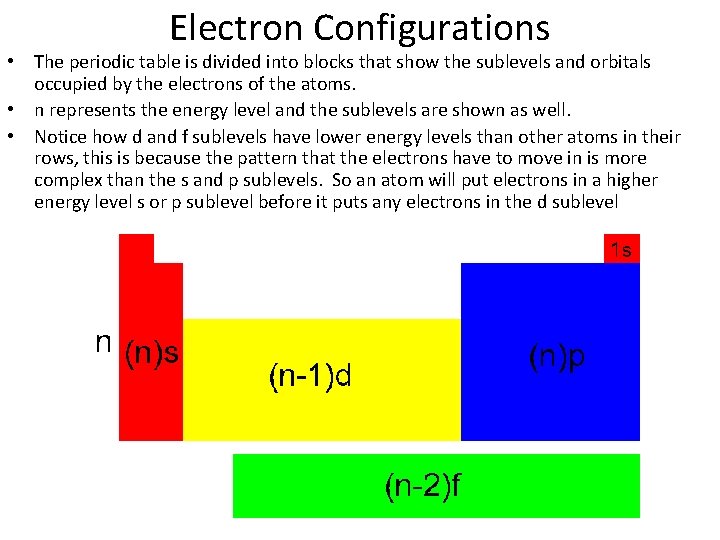
Electron Configurations • The periodic table is divided into blocks that show the sublevels and orbitals occupied by the electrons of the atoms. • n represents the energy level and the sublevels are shown as well. • Notice how d and f sublevels have lower energy levels than other atoms in their rows, this is because the pattern that the electrons have to move in is more complex than the s and p sublevels. So an atom will put electrons in a higher energy level s or p sublevel before it puts any electrons in the d sublevel

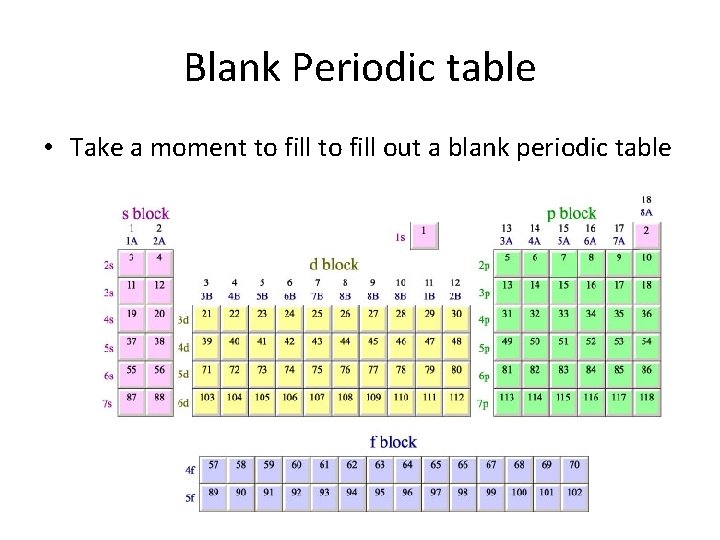
Blank Periodic table • Take a moment to fill out a blank periodic table

How to write an electron configuration • 1. Find the symbol for the element • 2. Write the symbol in brackets for the nearest smaller noble gas • 3 Write the outer electron configuration for the remaining electrons.

Practice Electron Configurations • • Li Be B C N O F Ne

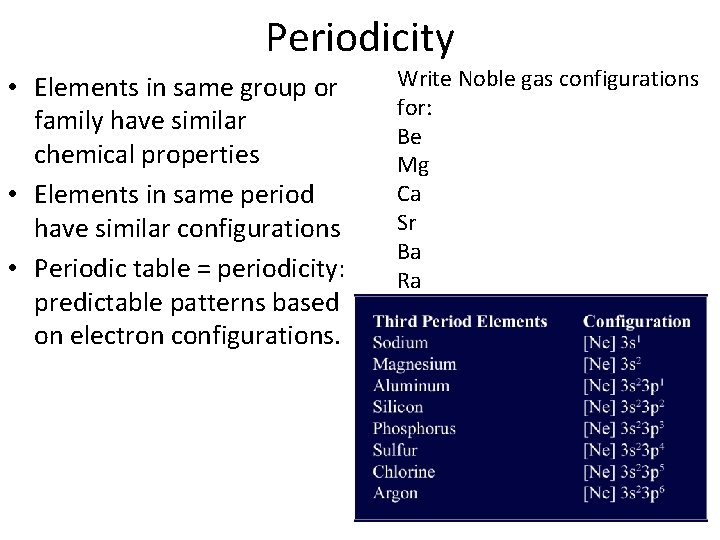
Periodicity • Elements in same group or family have similar chemical properties • Elements in same period have similar configurations • Periodic table = periodicity: predictable patterns based on electron configurations. Write Noble gas configurations for: Be Mg Ca Sr Ba Ra

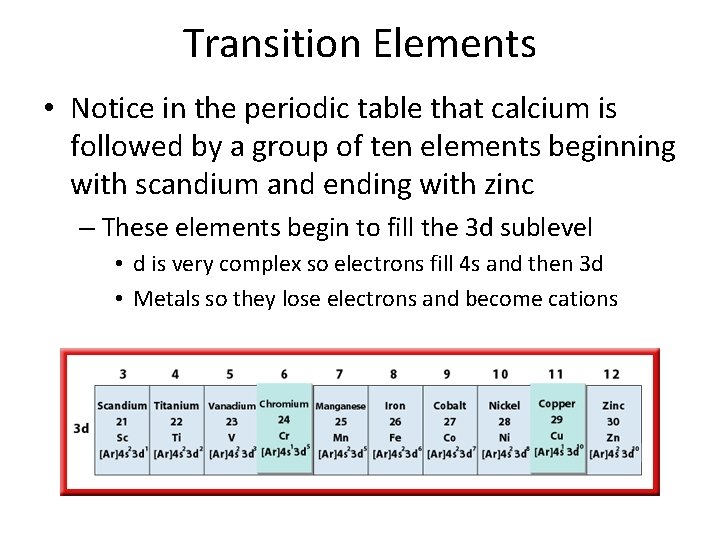
Transition Elements • Notice in the periodic table that calcium is followed by a group of ten elements beginning with scandium and ending with zinc – These elements begin to fill the 3 d sublevel • d is very complex so electrons fill 4 s and then 3 d • Metals so they lose electrons and become cations

Inner Transition Elements • Two rows at bottom of periodic table – lanthanides (atomic numbers 58 to 71) – actinides (atomic numbers 90 to 103) • These two series are called inner transition elements because their last electron occupies inner-level 4 f orbitals in the sixth period and the 5 f orbitals in the seventh period

An easy way to remember how atoms fill orbitals & sublevels