Light Light Terminology Which is not a measure



- Slides: 9

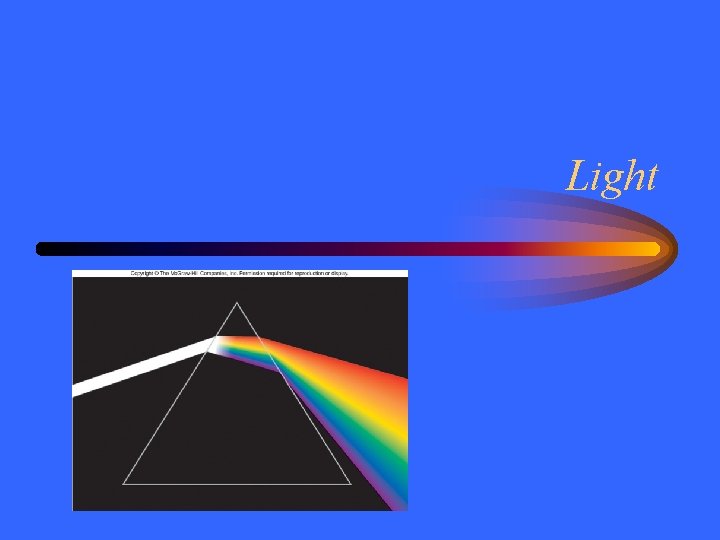
Light

Light Terminology Which is not a measure we use to identify a type of light? A. Wavelength B. Speed C. Frequency D. Energy

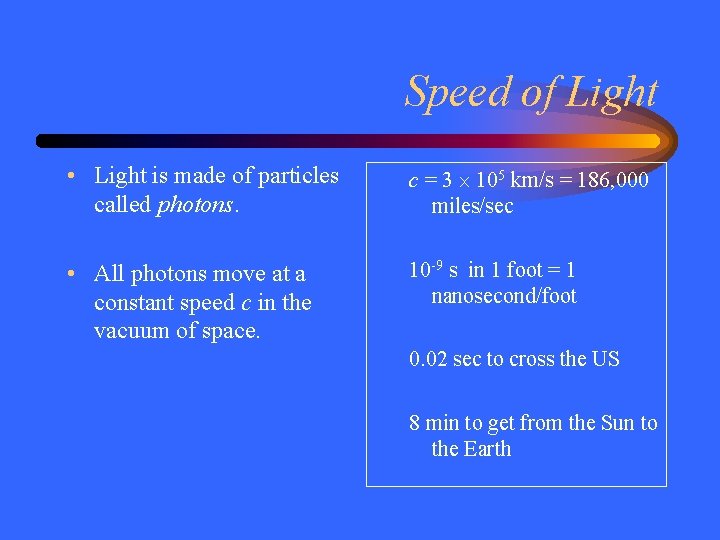
Speed of Light • Light is made of particles called photons. c = 3 105 km/s = 186, 000 miles/sec • All photons move at a constant speed c in the vacuum of space. 10 -9 s in 1 foot = 1 nanosecond/foot 0. 02 sec to cross the US 8 min to get from the Sun to the Earth

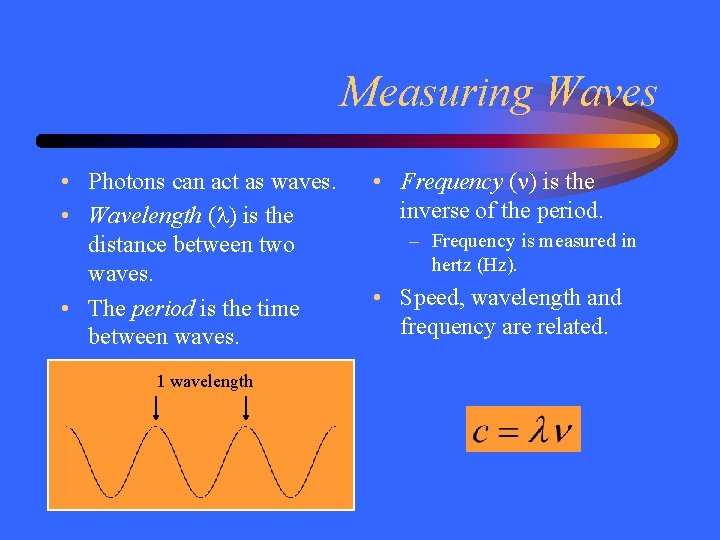
Measuring Waves • Photons can act as waves. • Wavelength ( ) is the distance between two waves. • The period is the time between waves. 1 wavelength • Frequency ( ) is the inverse of the period. – Frequency is measured in hertz (Hz). • Speed, wavelength and frequency are related.

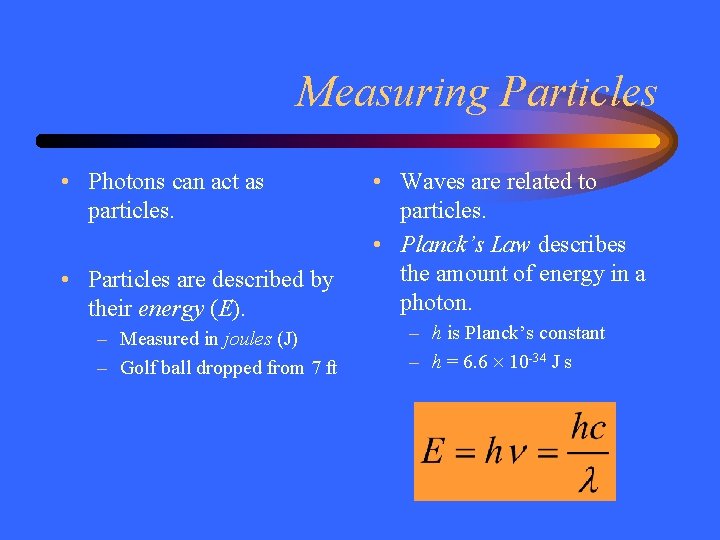
Measuring Particles • Photons can act as particles. • Particles are described by their energy (E). – Measured in joules (J) – Golf ball dropped from 7 ft • Waves are related to particles. • Planck’s Law describes the amount of energy in a photon. – h is Planck’s constant – h = 6. 6 10 -34 J s

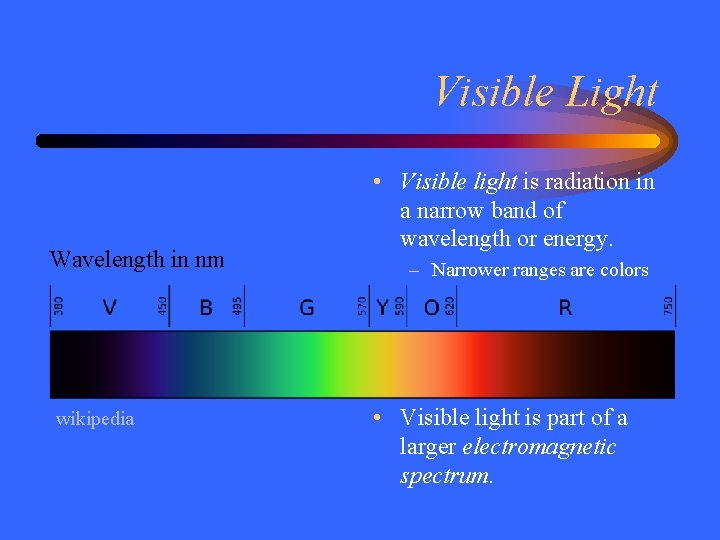
Visible Light Wavelength in nm wikipedia • Visible light is radiation in a narrow band of wavelength or energy. – Narrower ranges are colors • Visible light is part of a larger electromagnetic spectrum.

Radio Waves • Radio waves come from electrical oscillations. – Microwaves with shorter wavelengths • The long wavelengths travel easily and are not interfered by atoms. Radio waves > 1 m Microwaves 1 mm to 1 m < 3 x 108 Hz 3 x 1011 Hz to 3 x 108 Hz

Infrared and Ultraviolet • Visible light is often produced by vibrating atoms and molecules. – Infrared lower energy than visible light – Ultraviolet higher energy than visible light Infrared Visible light Ultraviolet 700 nm to 1 mm 400 nm to 700 nm 10 nm to 400 nm 4 x 1014 Hz to 3 x 1011 Hz 8 x 1014 Hz to 4 x 1014 Hz 3 x 1016 Hz to 8 x 1014 Hz

High Energy Photons • X-rays come from energetic transitions in atoms. • Gamma rays come from nuclear and subatomic reactions. X-rays 0. 01 to 10 nm Gamma rays < 0. 01 nm Thinkstock. com 3 x 1019 Hz to 3 x 1016 Hz > 3 x 1019 Hz