Light emissions minilab Pt 1 Flame test Wrap

Light emissions mini-lab Pt 1: Flame test Wrap up

Flame Test Lithium Sodium Potassium Electrons in the dissolved metal ions are excited by heat energy from the flame.
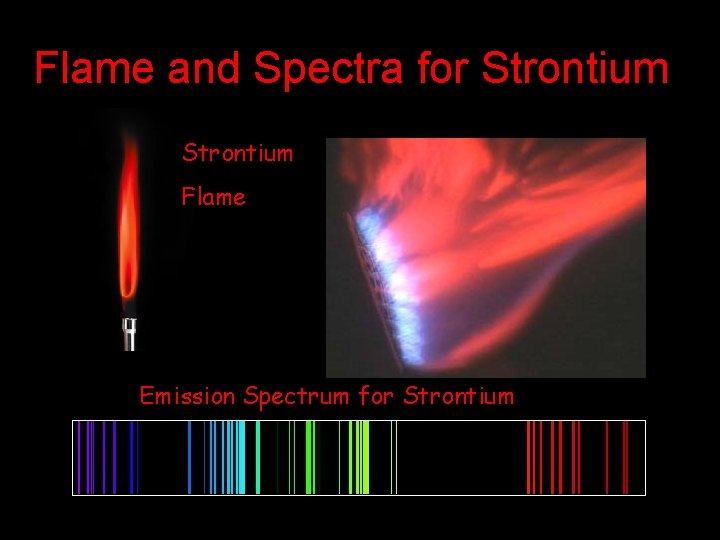
Flame and Spectra for Strontium Flame Emission Spectrum for Strontium
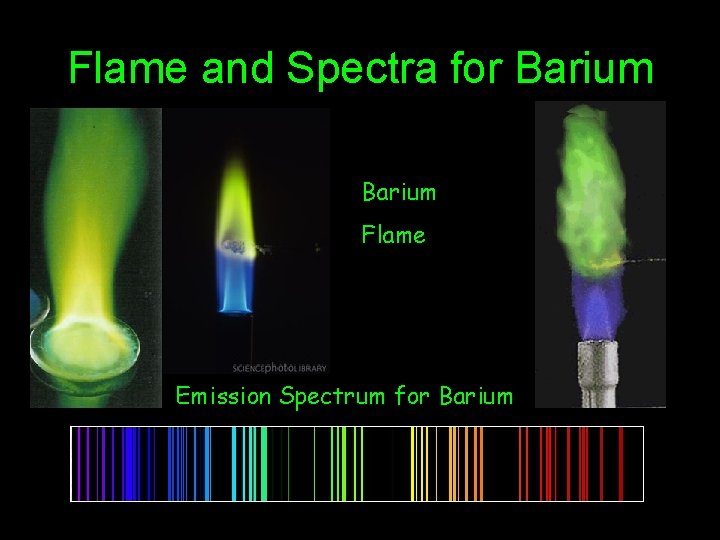
Flame and Spectra for Barium Flame Emission Spectrum for Barium
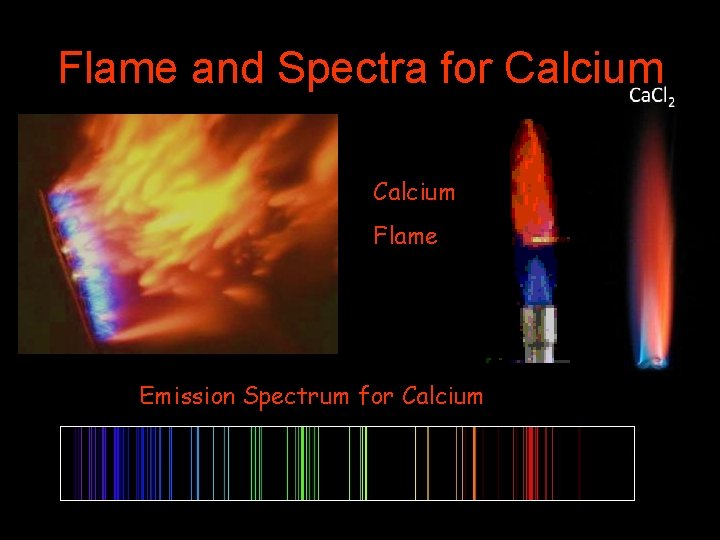
Flame and Spectra for Calcium Flame Emission Spectrum for Calcium

Flame and Spectra for Sodium Vapor Flame Streetlight Emission Spectrum for Sodium
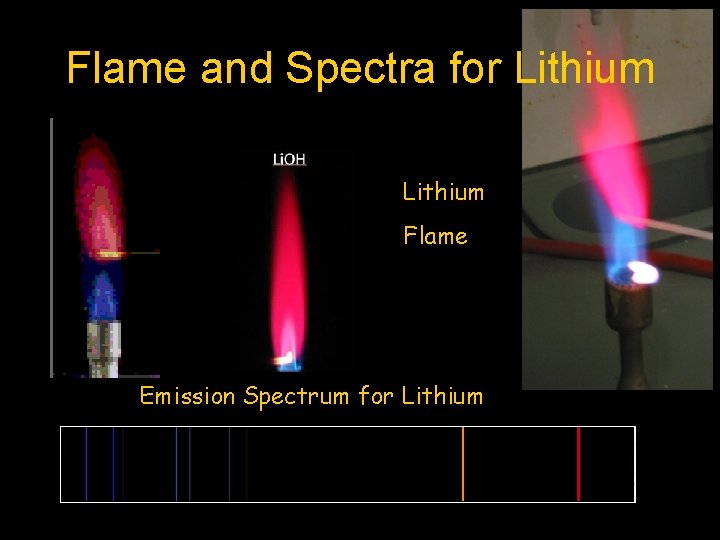
Flame and Spectra for Lithium Flame Emission Spectrum for Lithium
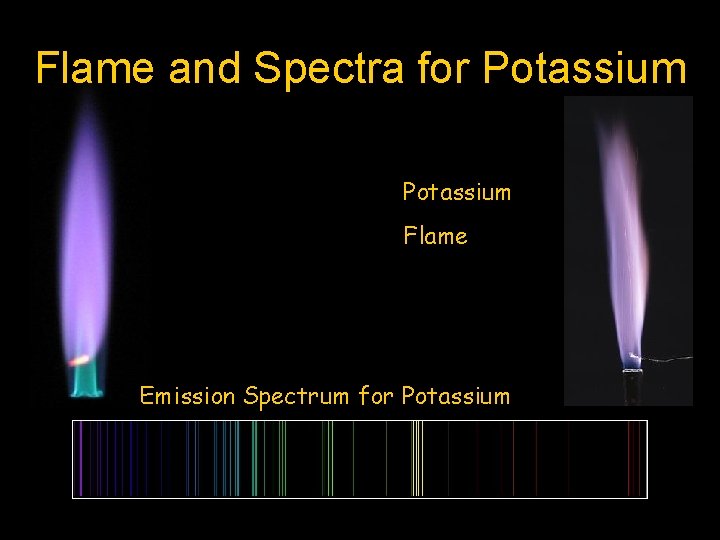
Flame and Spectra for Potassium Flame Emission Spectrum for Potassium
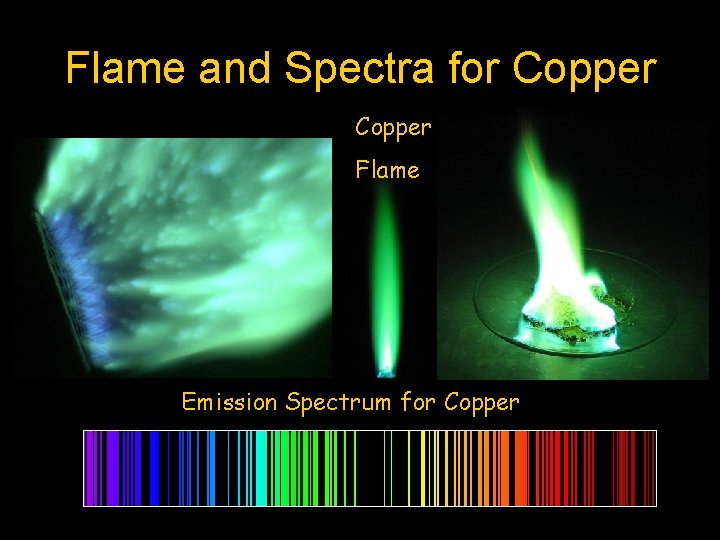
Flame and Spectra for Copper Flame Emission Spectrum for Copper

Comparing Unknown Flame to Known Flame Colors Sr Ba Ca Na Unknown Li K Cu Flame is ? ? ?

Light emissions mini-lab Pt 2: review of Bright Line Spectra
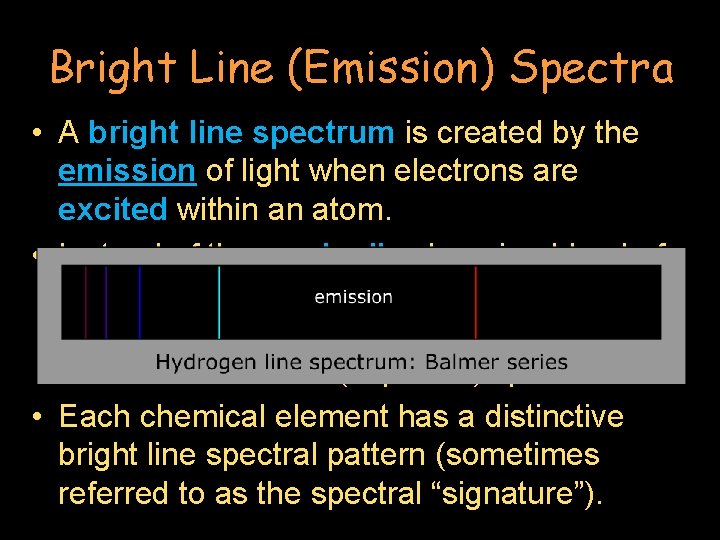
Bright Line (Emission) Spectra • A bright line spectrum is created by the emission of light when electrons are excited within an atom. • Instead of the gradually changing blend of colors found in a continuous spectrum, there anywhere from a handful to dozens of discrete (separate) spectral lines. • Each chemical element has a distinctive bright line spectral pattern (sometimes referred to as the spectral “signature”).
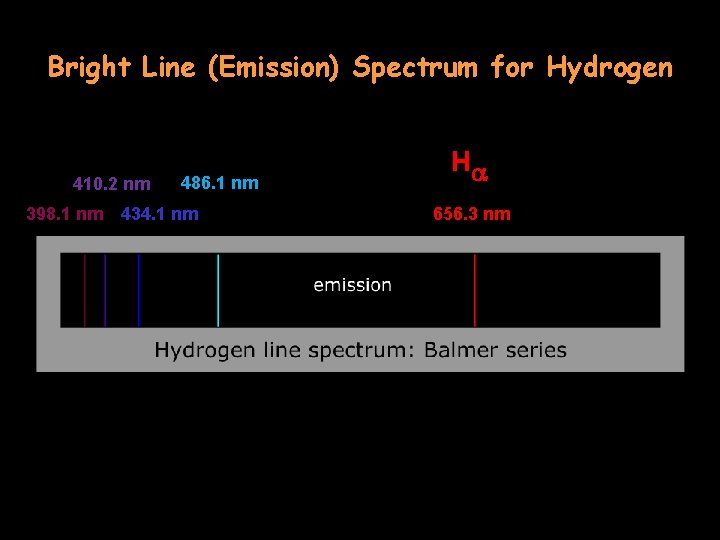
Bright Line (Emission) Spectrum for Hydrogen 410. 2 nm 398. 1 nm 486. 1 nm 434. 1 nm Ha 656. 3 nm
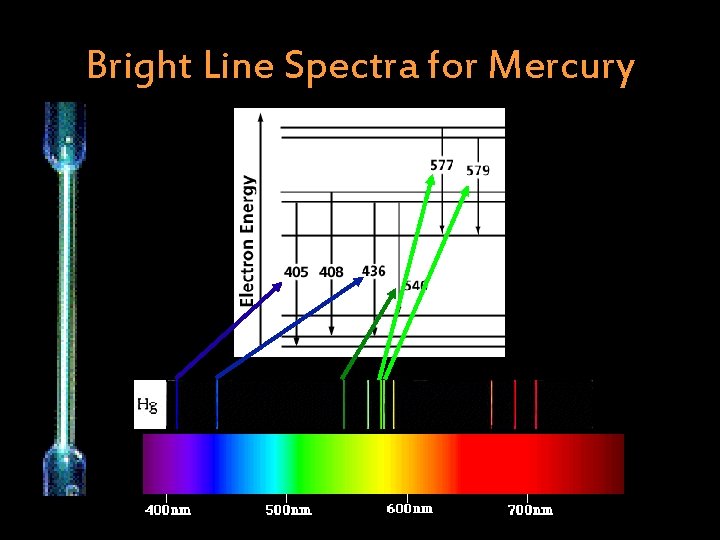
Bright Line Spectra for Mercury
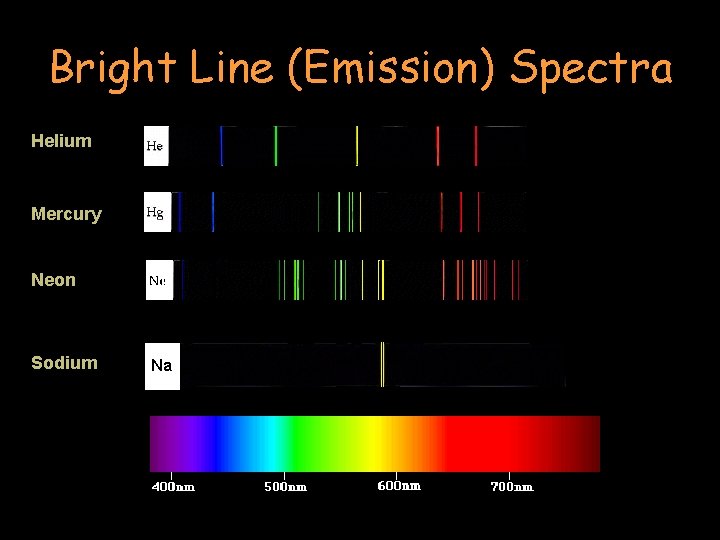
Bright Line (Emission) Spectra Helium Mercury Neon Sodium Na
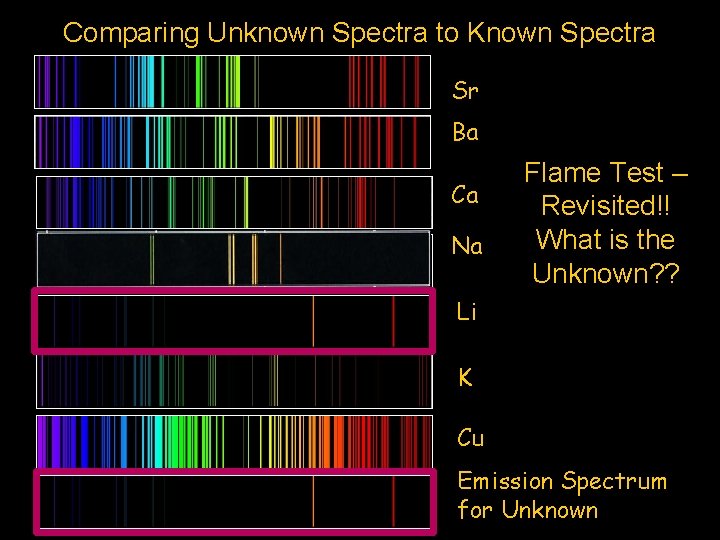
Comparing Unknown Spectra to Known Spectra Sr Ba Ca Na Flame Test – Revisited!! What is the Unknown? ? Li K Cu Emission Spectrum for Unknown
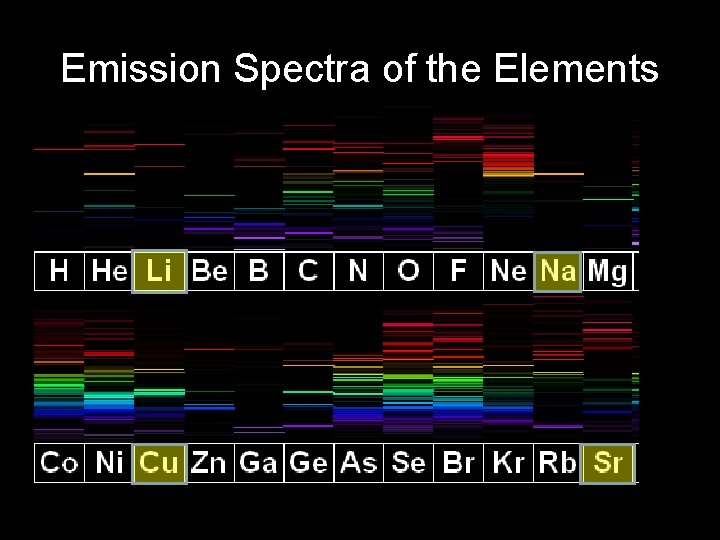
Emission Spectra of the Elements
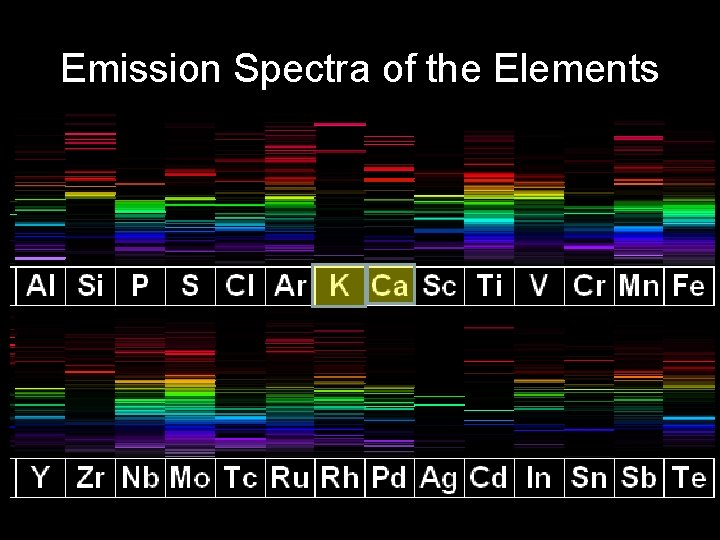
Emission Spectra of the Elements
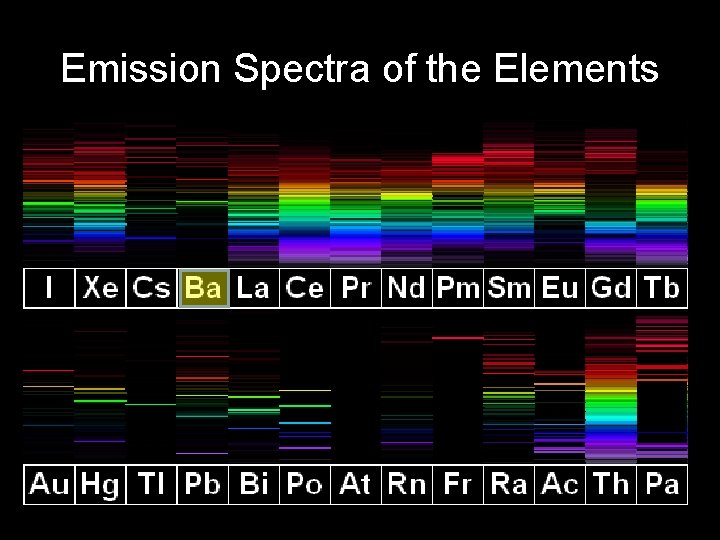
Emission Spectra of the Elements
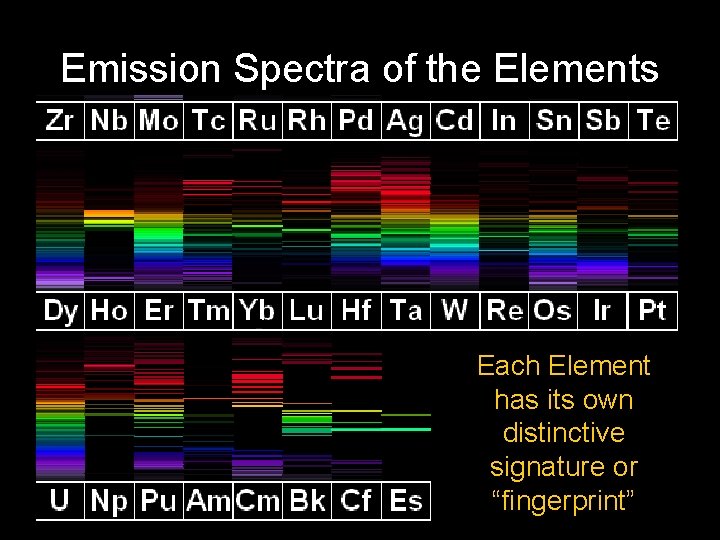
Emission Spectra of the Elements Each Element has its own distinctive signature or “fingerprint”

Fireworks – Phosphorus, Magnesium Electrons in fireworks are excited by heat energy from the explosive chemical reactions.

Neon Signs and Spectrum Tubes Electrons in gas tubes are excited by high voltage electricity.

Emission and Planetary Nebulae Electrons in nebular gases are excited by high energy ultraviolet radiation from nearby stars.

Pt 3 Incandescent objects and Black Body Radiation
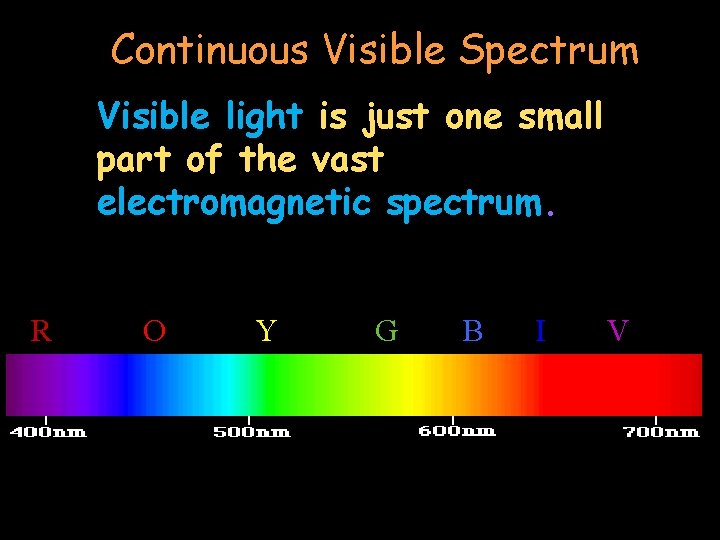
Continuous Visible Spectrum Visible light is just one small part of the vast electromagnetic spectrum. R O Y G B I V
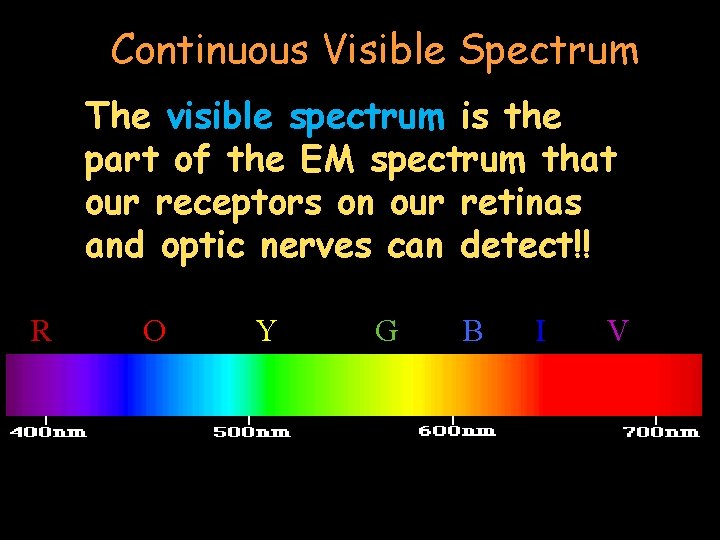
Continuous Visible Spectrum The visible spectrum is the part of the EM spectrum that our receptors on our retinas and optic nerves can detect!! R O Y G B I V
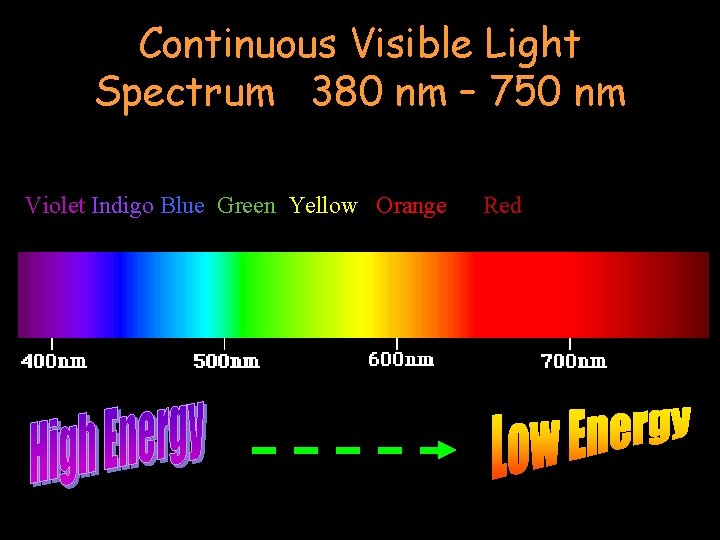
Continuous Visible Light Spectrum 380 nm – 750 nm Violet Indigo Blue Green Yellow Orange Red

Blackbody Radiation Max Planck noticed that incandescent - hot, glowing solids (such as iron and the tungsten filament in a light bulb) changed colors as they got hotter: black red hot yellow hot white hot blue hot

Blackbody Radiation (As the object gets hotter, wavelength gets shorter. ) So red hot is not really all that hot!! The largest incandescent objects in the universe are stars: black red hot yellow hot white hot blue hot
Incandescent Metals Temperature Color
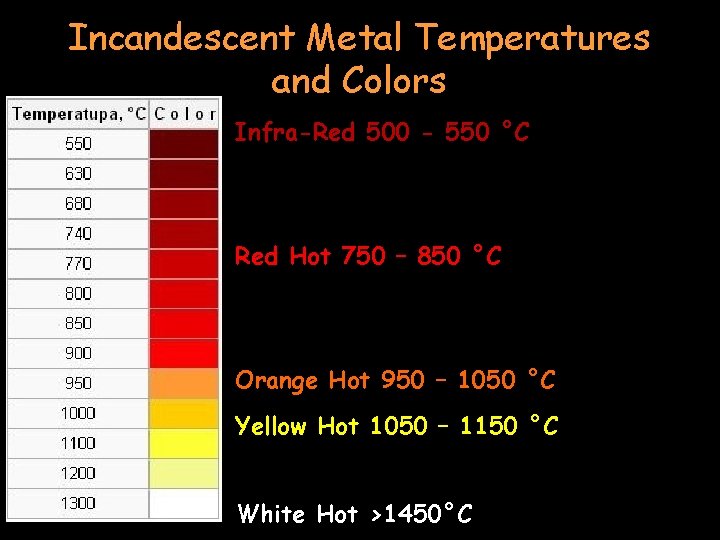
Incandescent Metal Temperatures and Colors Infra-Red 500 - 550 °C Red Hot 750 – 850 °C Orange Hot 950 – 1050 °C Yellow Hot 1050 – 1150 °C White Hot >1450°C
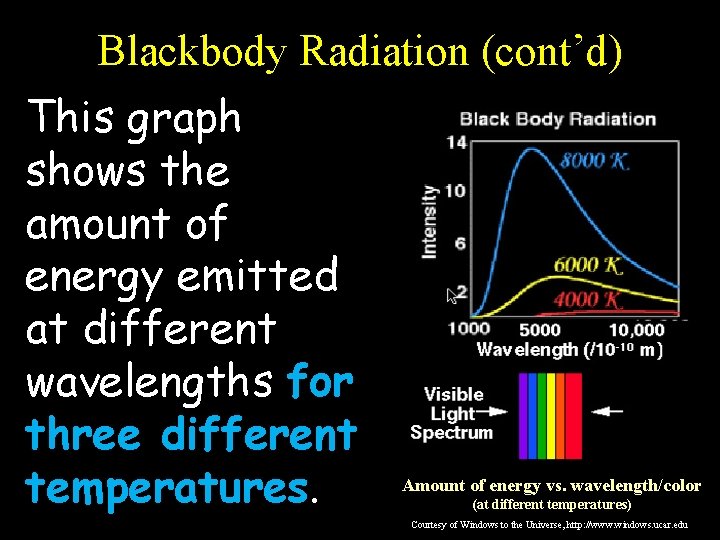
Blackbody Radiation (cont’d) This graph shows the amount of energy emitted at different wavelengths for three different temperatures. Amount of energy vs. wavelength/color (at different temperatures) Courtesy of Windows to the Universe, http: //www. windows. ucar. edu
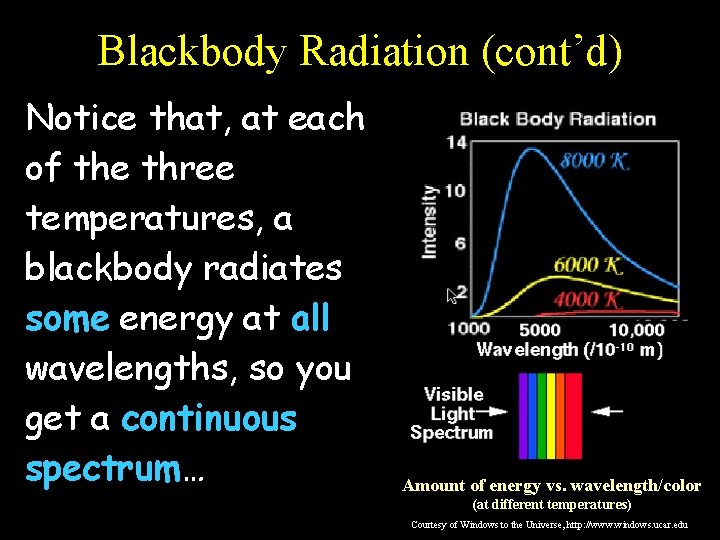
Blackbody Radiation (cont’d) Notice that, at each of the three temperatures, a blackbody radiates some energy at all wavelengths, so you get a continuous spectrum… Amount of energy vs. wavelength/color (at different temperatures) Courtesy of Windows to the Universe, http: //www. windows. ucar. edu
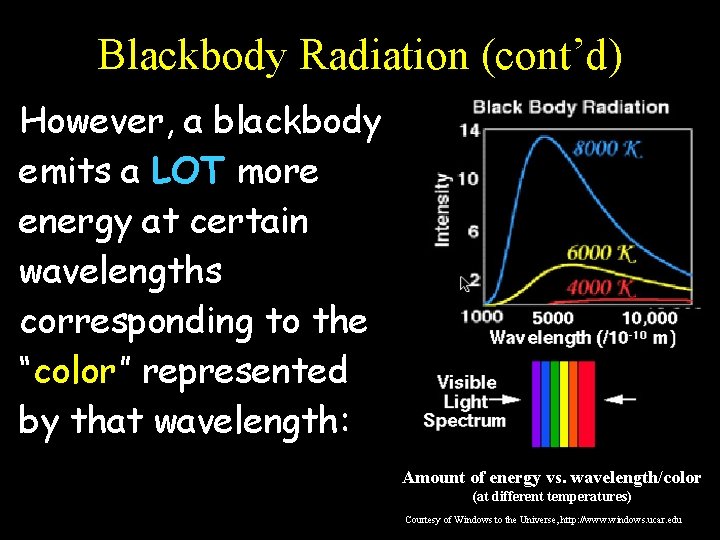
Blackbody Radiation (cont’d) However, a blackbody emits a LOT more energy at certain wavelengths corresponding to the “color” represented by that wavelength: Amount of energy vs. wavelength/color (at different temperatures) Courtesy of Windows to the Universe, http: //www. windows. ucar. edu
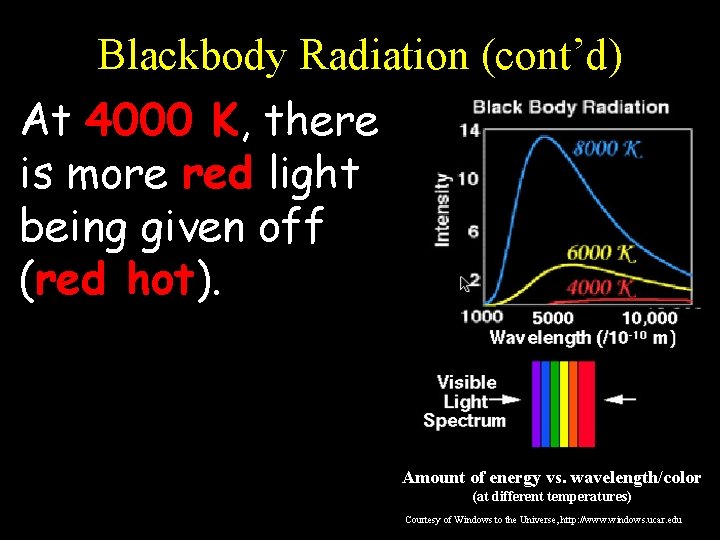
Blackbody Radiation (cont’d) At 4000 K, there is more red light being given off (red hot). Amount of energy vs. wavelength/color (at different temperatures) Courtesy of Windows to the Universe, http: //www. windows. ucar. edu
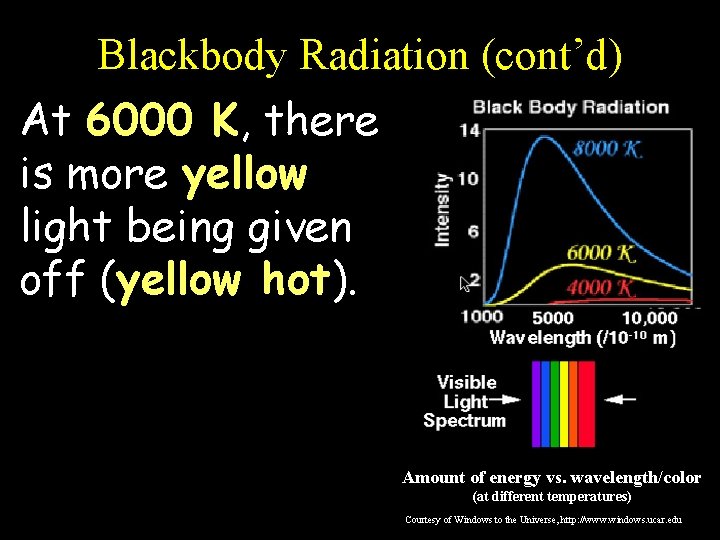
Blackbody Radiation (cont’d) At 6000 K, there is more yellow light being given off (yellow hot). Amount of energy vs. wavelength/color (at different temperatures) Courtesy of Windows to the Universe, http: //www. windows. ucar. edu
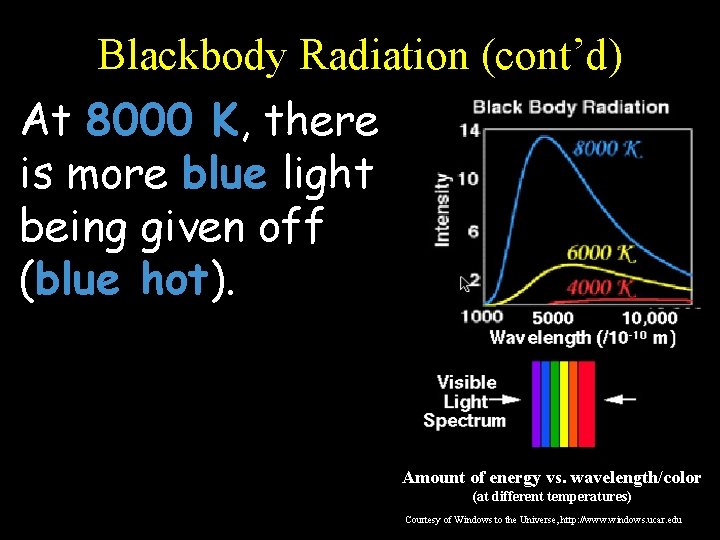
Blackbody Radiation (cont’d) At 8000 K, there is more blue light being given off (blue hot). Amount of energy vs. wavelength/color (at different temperatures) Courtesy of Windows to the Universe, http: //www. windows. ucar. edu
Blackbody Radiation Incandescent objects such as stars are called “blackbodies”. blackbodies blackbody: A blackbody: blackbody • absorbs ALL radiation – molecules move faster when heated • reradiates ALL that energy back out into the environment • creates a continuous spectrum of light • radiates much more energy as it gets hotter.

Blackbody Radiation • as it gets hotter, wavelengths ( l ) get shorter & shorter: • Black Red Hot Yellow Hot White Hot Blue Hot • (Explains why blue giant stars are really hot and red giant stars are relatively cool. )
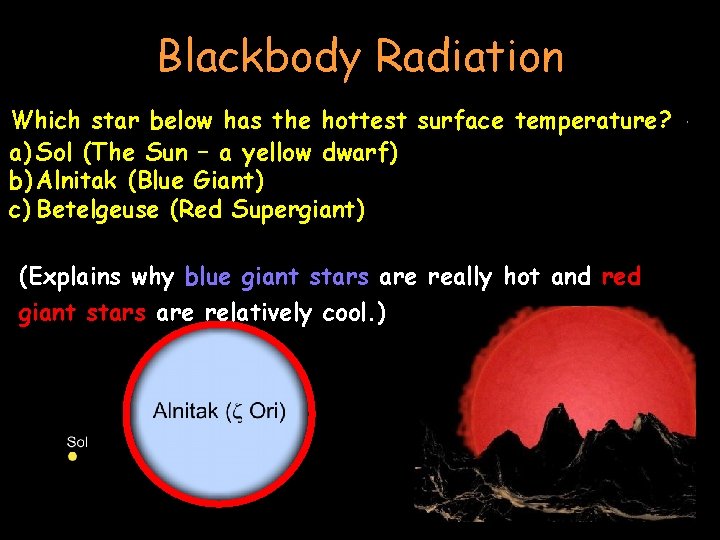
Blackbody Radiation Which below hashotter, the hottest surface temperature? As thestar object gets the wavelength gets shortera) Sol Sunis– not a yellow So (The red hot reallydwarf) all that hot!! b) Alnitak (Blue Giant) largest incandescent objects in the universe are c)The Betelgeuse (Red Supergiant) stars. (Explains why blue giant stars are really hot and red giant stars are relatively cool. )

Pt 4. . . back to spectral classes of stars !!
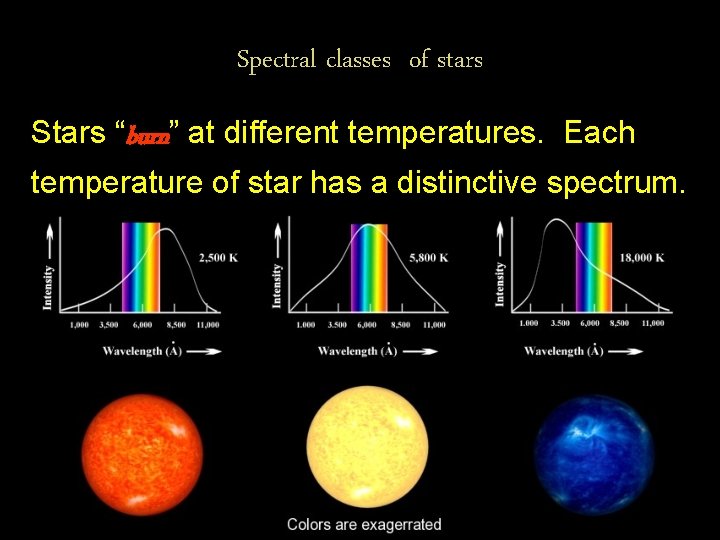
Spectral classes of stars Stars “burn” at different temperatures. Each temperature of star has a distinctive spectrum.

Harvard Spectral classification of stars • Class O - Blue (super hot - 41, 000 o K) (Range = 28, 000 - 50, 000 o K) • Class B - Blue/White (Range = 10, 000 - 28, 000 o K) • Class A - White (Range = 7, 500 - 10, 000 o K) • Class F - Yellow-White (Range = 6, 000 - 7, 500 o K)
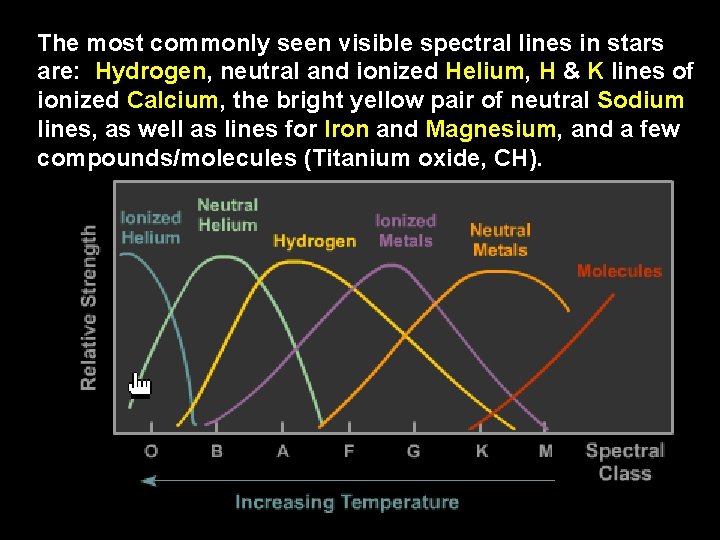
The most commonly seen visible spectral lines in stars are: Hydrogen, neutral and ionized Helium, H & K lines of ionized Calcium, the bright yellow pair of neutral Sodium lines, as well as lines for Iron and Magnesium, and a few compounds/molecules (Titanium oxide, CH).
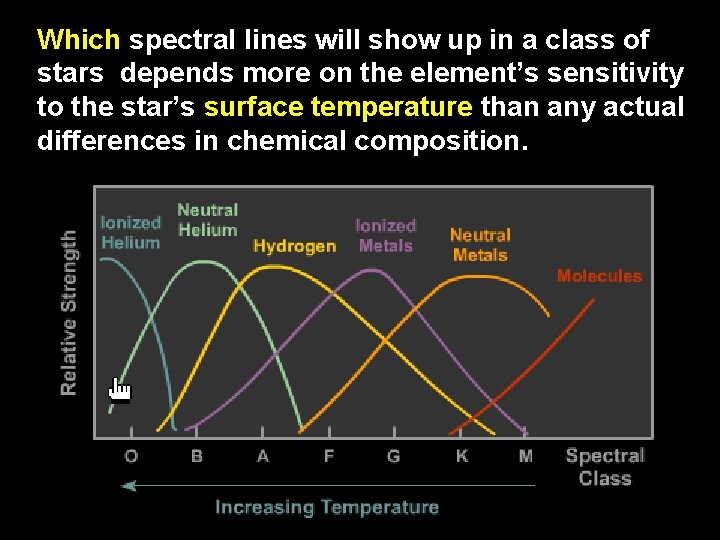
Which spectral lines will show up in a class of stars depends more on the element’s sensitivity to the star’s surface temperature than any actual differences in chemical composition.
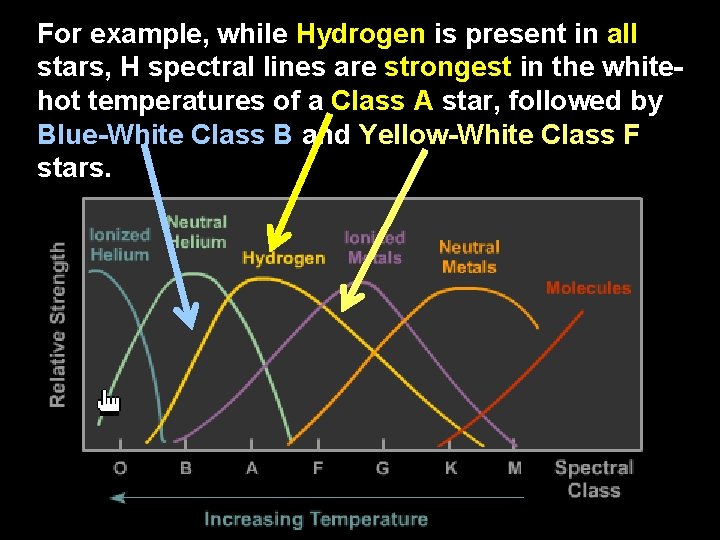
For example, while Hydrogen is present in all stars, H spectral lines are strongest in the whitehot temperatures of a Class A star, followed by Blue-White Class B and Yellow-White Class F stars.
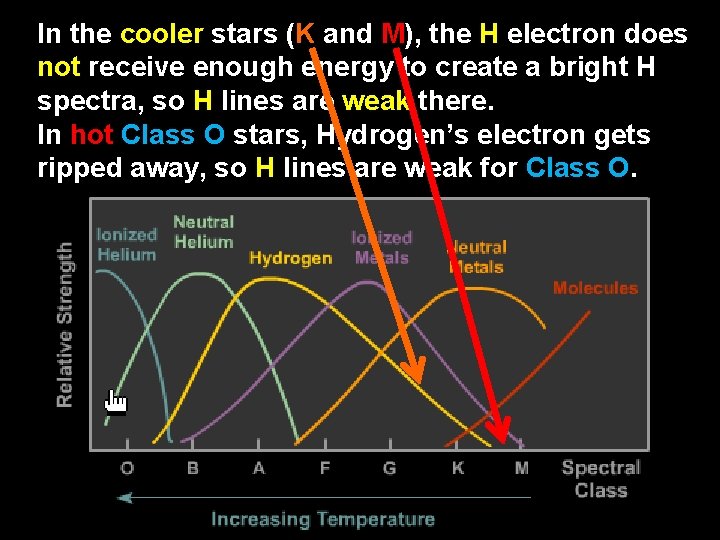
In the cooler stars (K and M), the H electron does not receive enough energy to create a bright H spectra, so H lines are weak there. In hot Class O stars, Hydrogen’s electron gets ripped away, so H lines are weak for Class O.
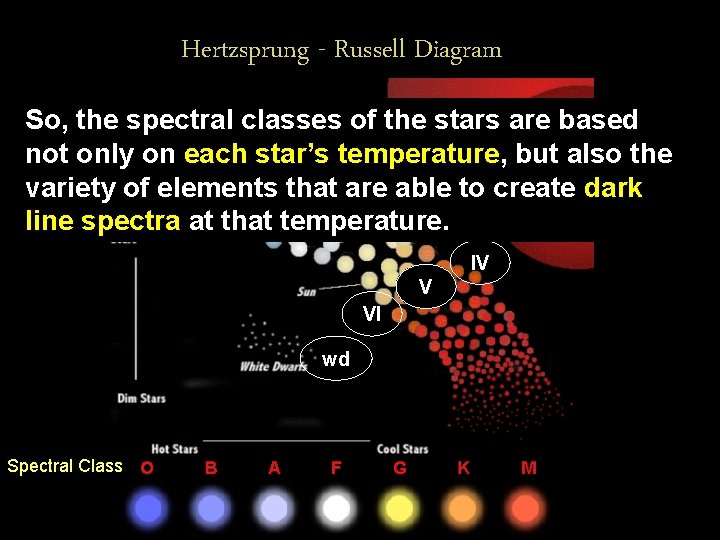
Hertzsprung - Russell Diagram So, Sequence the spectral Main Stars classes of the stars are based I also the not only on each star’s temperature, but II variety of elements that are able to create dark line spectra at that temperature. III IV V VI wd Spectral Class

Pt 5 dark line spectra of stars

Corona of the Sun Cooling gases in the sun’s outer corona absorb certain frequencies of solar radiation.
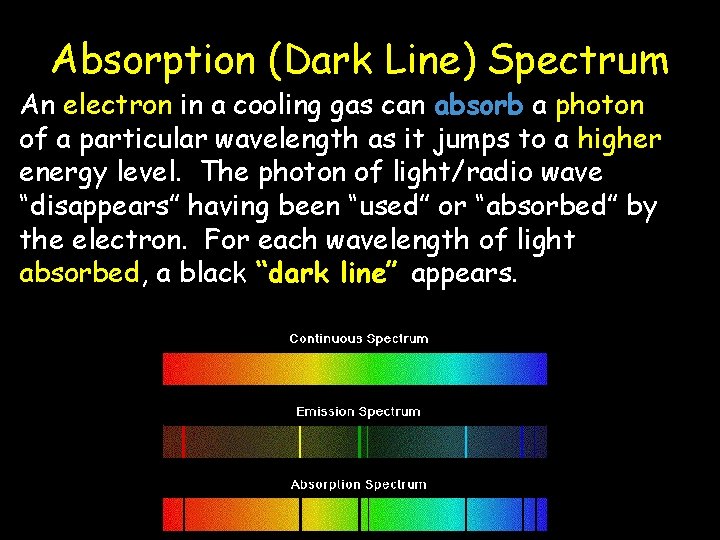
Absorption (Dark Line) Spectrum An electron in a cooling gas can absorb a photon of a particular wavelength as it jumps to a higher energy level. The photon of light/radio wave “disappears” having been “used” or “absorbed” by the electron. For each wavelength of light absorbed, a black “dark line” appears.
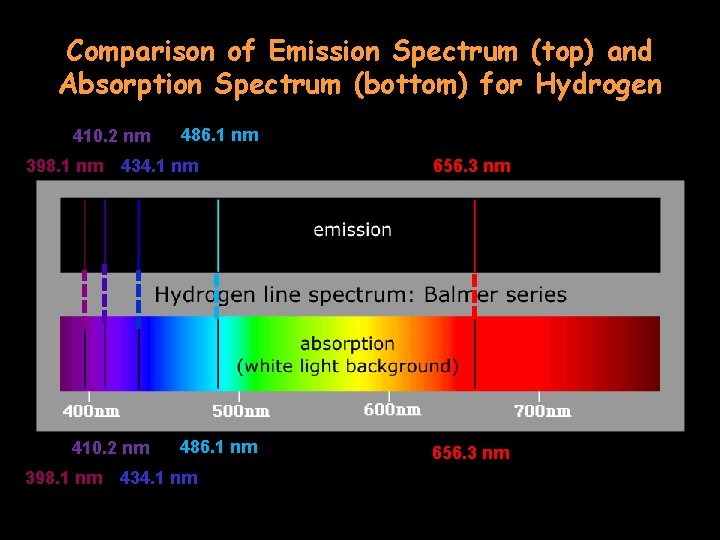
Comparison of Emission Spectrum (top) and Absorption Spectrum (bottom) for Hydrogen 410. 2 nm 398. 1 nm 434. 1 nm 410. 2 nm 398. 1 nm 486. 1 nm 434. 1 nm 656. 3 nm
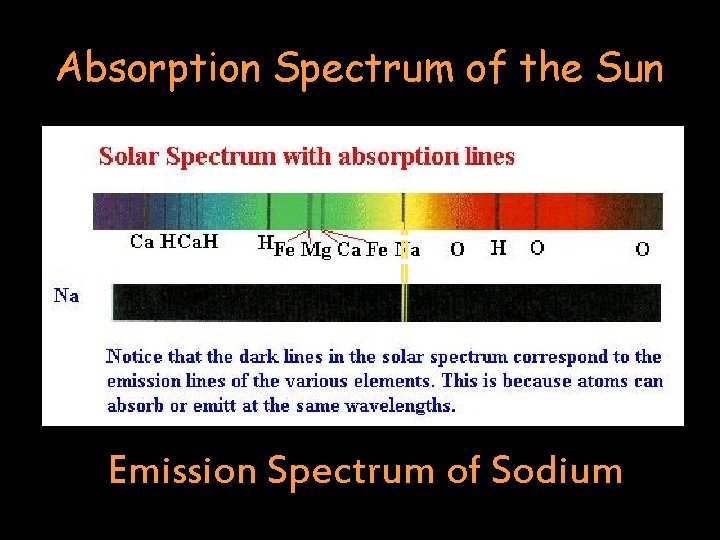
Absorption Spectrum of the Sun Emission Spectrum of Sodium
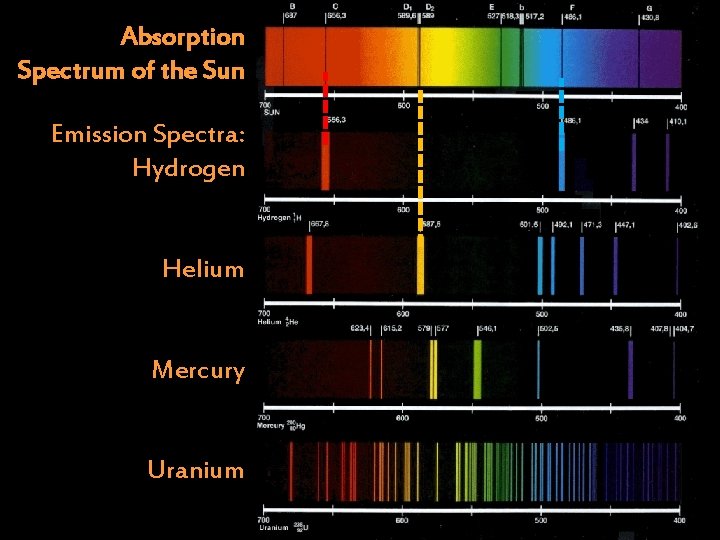
Absorption Spectrum of the Sun Emission Spectra: Hydrogen Helium Mercury Uranium
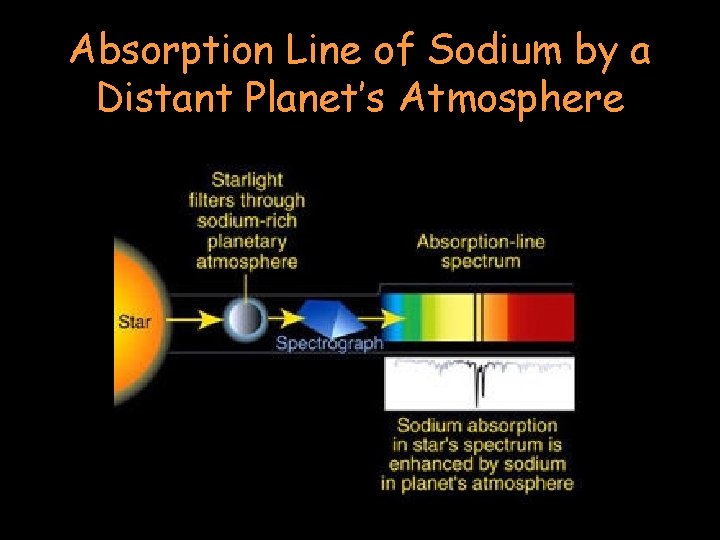
Absorption Line of Sodium by a Distant Planet’s Atmosphere
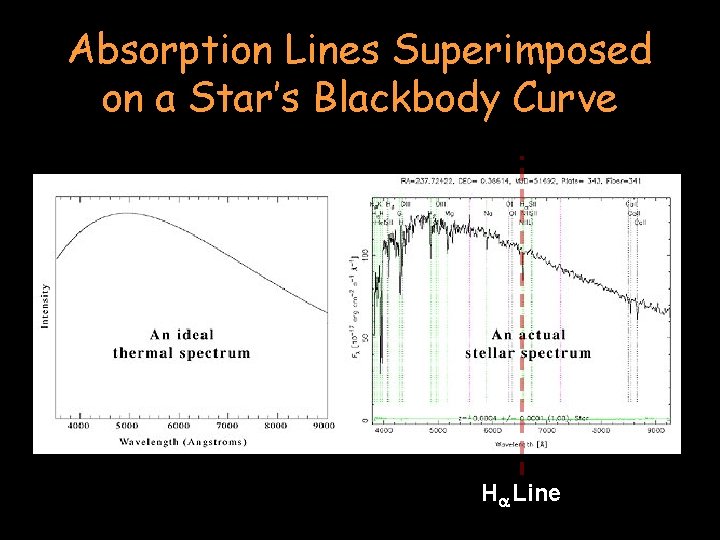
Absorption Lines Superimposed on a Star’s Blackbody Curve Ha Line
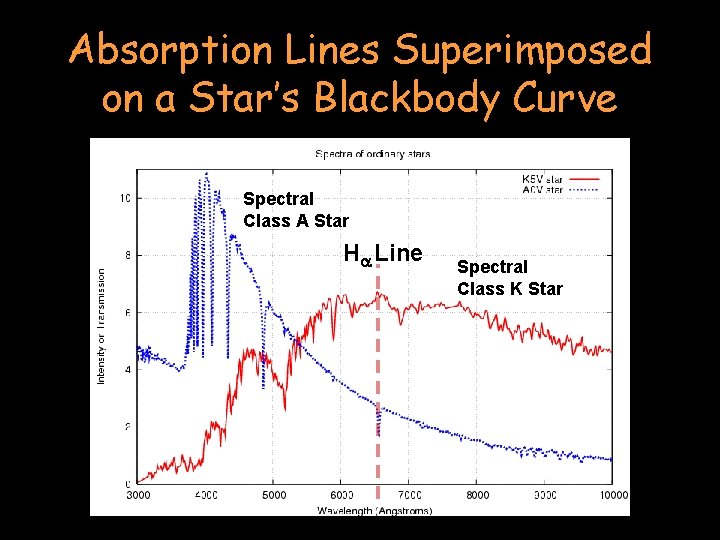
Absorption Lines Superimposed on a Star’s Blackbody Curve Spectral Class A Star Ha Line Spectral Class K Star
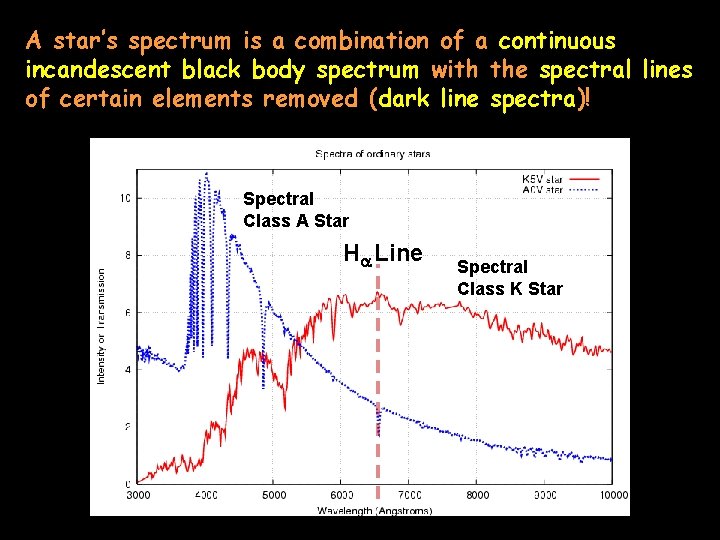
A star’s spectrum is a combination of a continuous incandescent black body spectrum with the spectral lines of certain elements removed (dark line spectra)! Spectral Class A Star Ha Line Spectral Class K Star
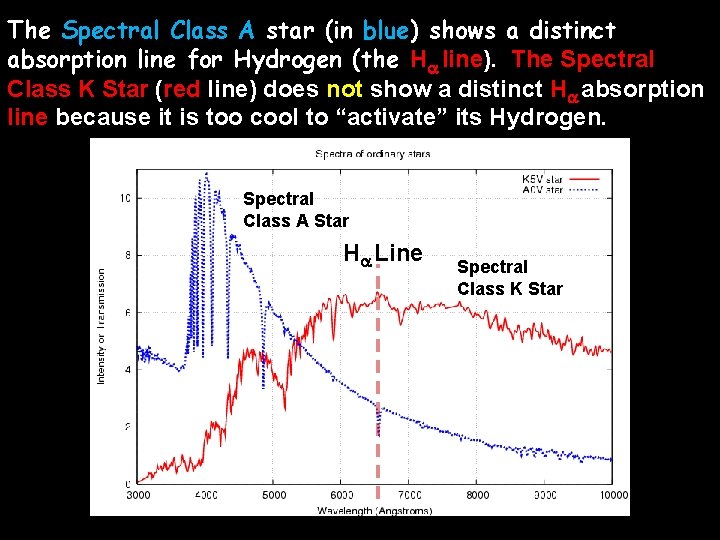
The Spectral Class A star (in blue) shows a distinct absorption line for Hydrogen (the Ha line). The Spectral Class K Star (red line) does not show a distinct Ha absorption line because it is too cool to “activate” its Hydrogen. Spectral Class A Star Ha Line Spectral Class K Star

The End!!
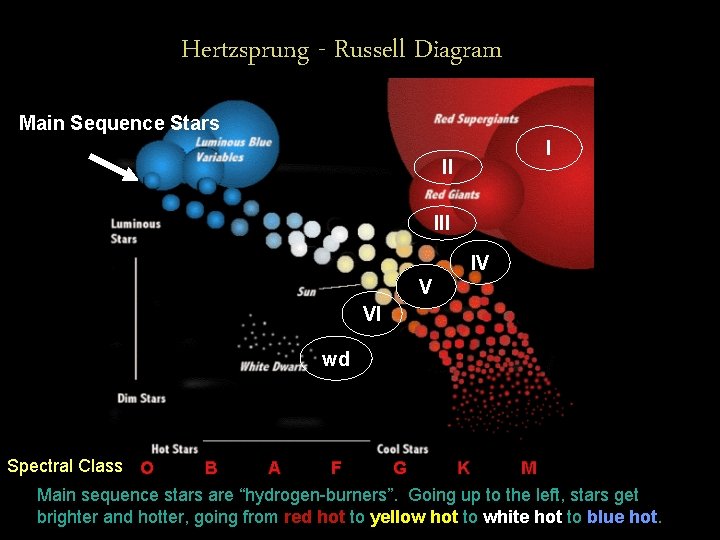
Hertzsprung - Russell Diagram Main Sequence Stars I II IV V VI wd Spectral Class Main sequence stars are “hydrogen-burners”. Going up to the left, stars get brighter and hotter, going from red hot to yellow hot to white hot to blue hot.
- Slides: 61