Light and The Electromagnetic Spectrum Why do we












- Slides: 28

Light and The Electromagnetic Spectrum

Why do we have to study “light”? . . . Because almost everything in astronomy is known because of light (or some other form of electromagnetic wave) coming from stars, planets, galaxies, etc.

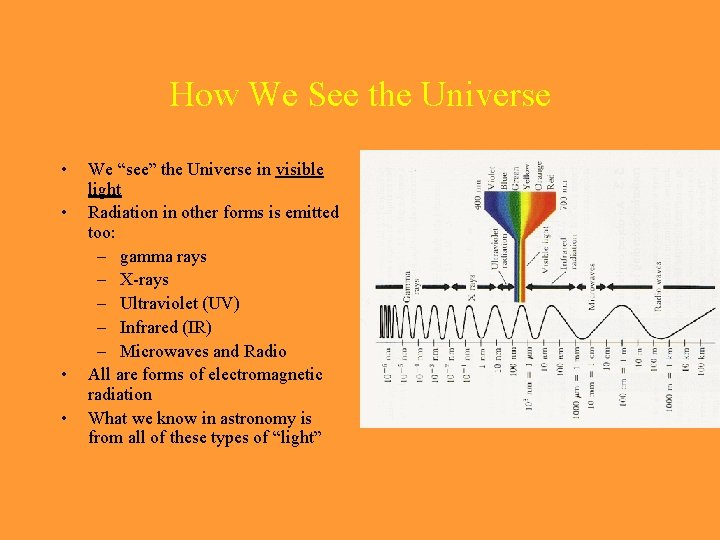
How We See the Universe • • We “see” the Universe in visible light Radiation in other forms is emitted too: – gamma rays – X-rays – Ultraviolet (UV) – Infrared (IR) – Microwaves and Radio All are forms of electromagnetic radiation What we know in astronomy is from all of these types of “light”

Electromagnetic Waves • EM Waves are a response to changes in electrical and/or magnetic fields elsewhere. • EM waves do NOT need a medium to travel through

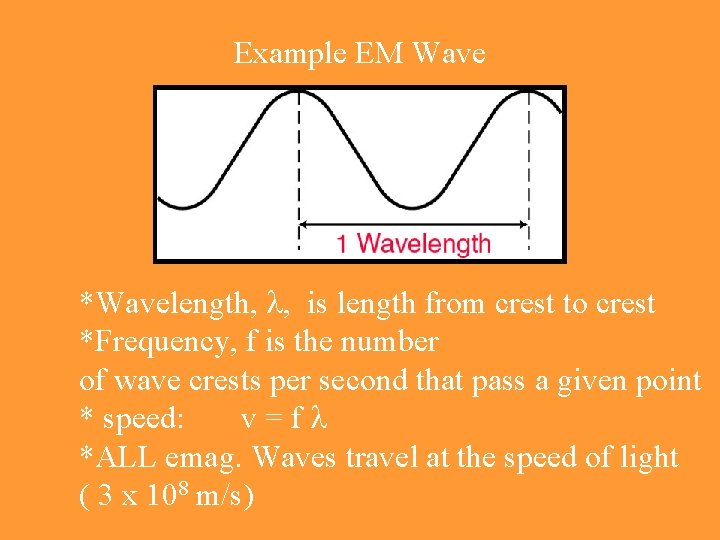
Example EM Wave *Wavelength, , is length from crest to crest *Frequency, f is the number of wave crests per second that pass a given point * speed: v=f *ALL emag. Waves travel at the speed of light ( 3 x 108 m/s)

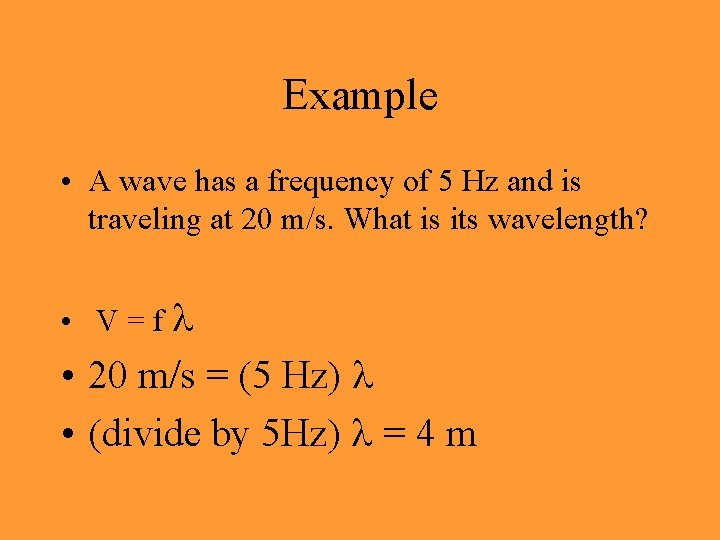
Example • A wave has a frequency of 5 Hz and is traveling at 20 m/s. What is its wavelength? • V=f • 20 m/s = (5 Hz) • (divide by 5 Hz) = 4 m

Example • A yellow light wave with a frequency of 5 x 1014 Hz. What is the wavelength of this yellow light? • (all emag waves travel at speed of light) • V=f • 3 x 108 m/s = (5 x 1014 Hz) • (divide by 5 x 1014 Hz) • = 6 x 10 -7 m

Electromagnetic Spectrum • Isaac Newton showed that ordinary sunlight could be split into many colors • Each color corresponds to light of a specific wavelength (or frequency)

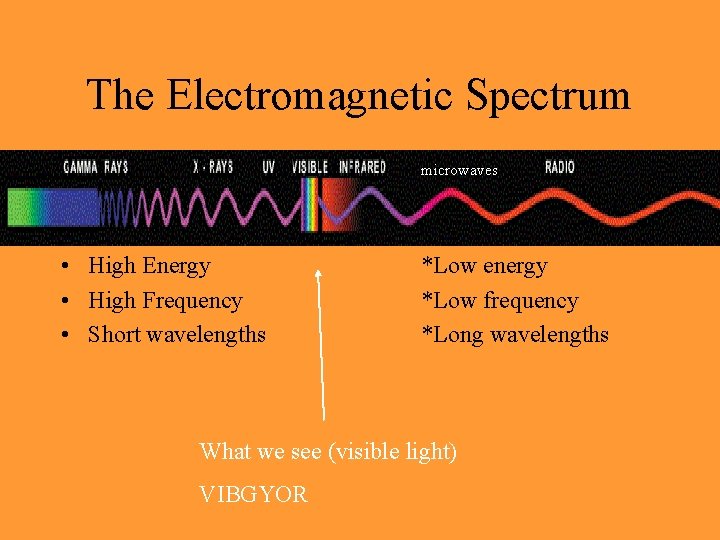
The Electromagnetic Spectrum microwaves • High Energy • High Frequency • Short wavelengths *Low energy *Low frequency *Long wavelengths What we see (visible light) VIBGYOR

Our sun at different wavelengths • http: //coolcosmos. ipac. caltech. edu/cosmic_ classroom/multiwavelength_astronomy/mul tiwavelength_museum/sun. html • Different parts of the sun will produce different wavelengths of electromagnetic radiation

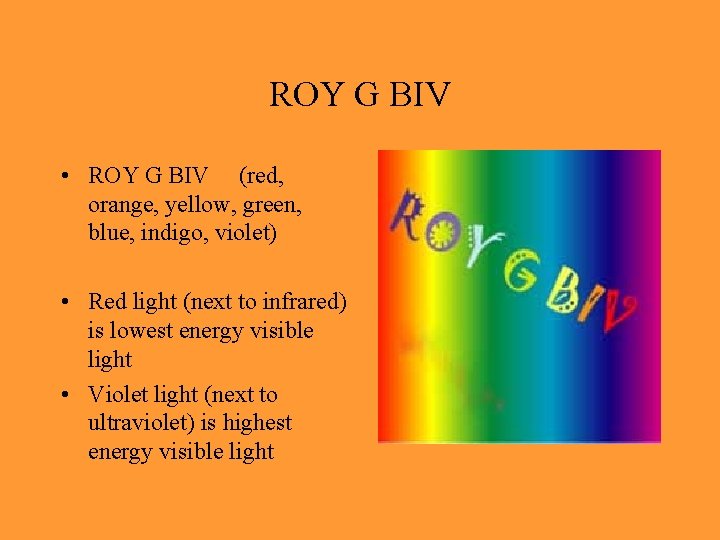
ROY G BIV • ROY G BIV (red, orange, yellow, green, blue, indigo, violet) • Red light (next to infrared) is lowest energy visible light • Violet light (next to ultraviolet) is highest energy visible light

“Brightness” • Flux (rate of energy per area) falls off according to the inverse square law • example: • Two light bulbs (A&B) are equally as bright. Bulb “B” is placed 3 times further away. How does its brightness compare? • Brightness 1/d 2 • Brightness 1/32 = 1/9 as bright

Doppler Effect • Motion of an object that emits or absorbs light causes a shift in the observed spectrum • Receding objects: spectrum ‘red-shifts’, so observed wavelength longer than normal • Approaching objects: spectrum ‘blue-shifts’, so observed wavelength is shorter than normal

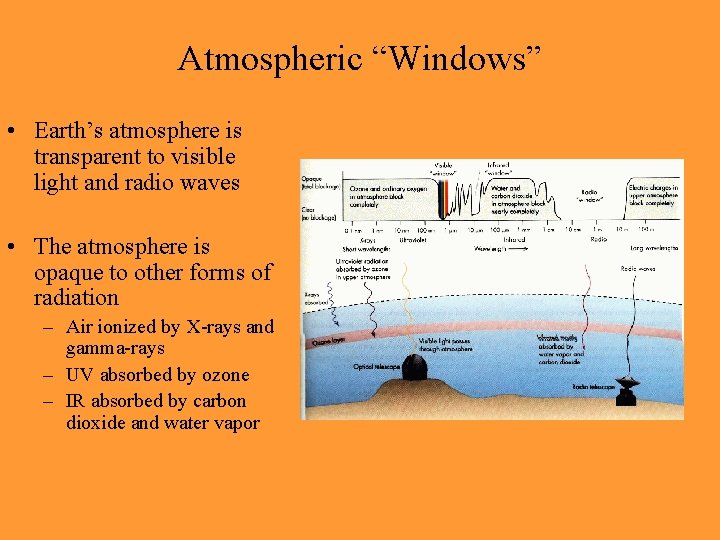
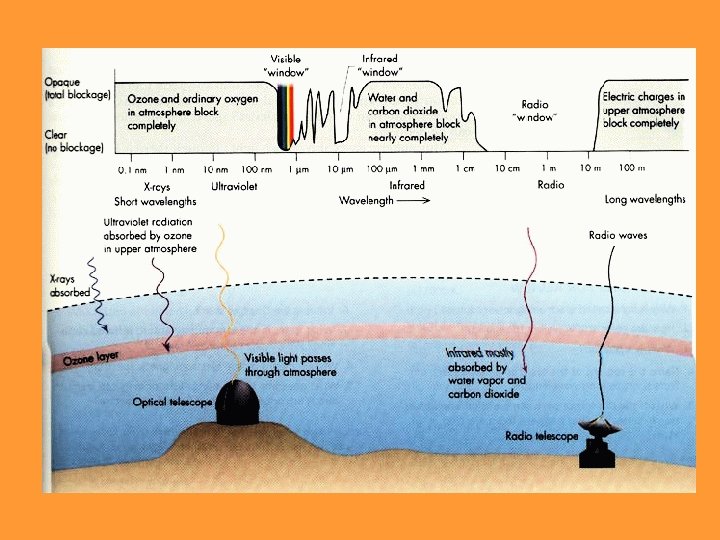
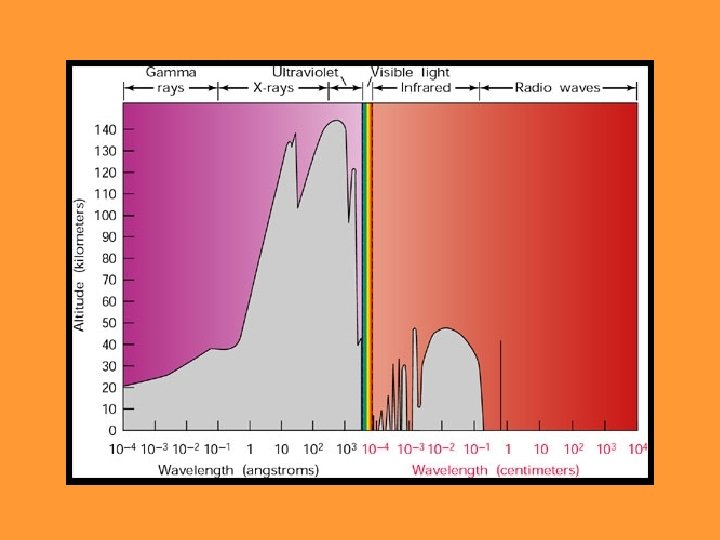
Atmospheric “Windows” • Earth’s atmosphere is transparent to visible light and radio waves • The atmosphere is opaque to other forms of radiation – Air ionized by X-rays and gamma-rays – UV absorbed by ozone – IR absorbed by carbon dioxide and water vapor



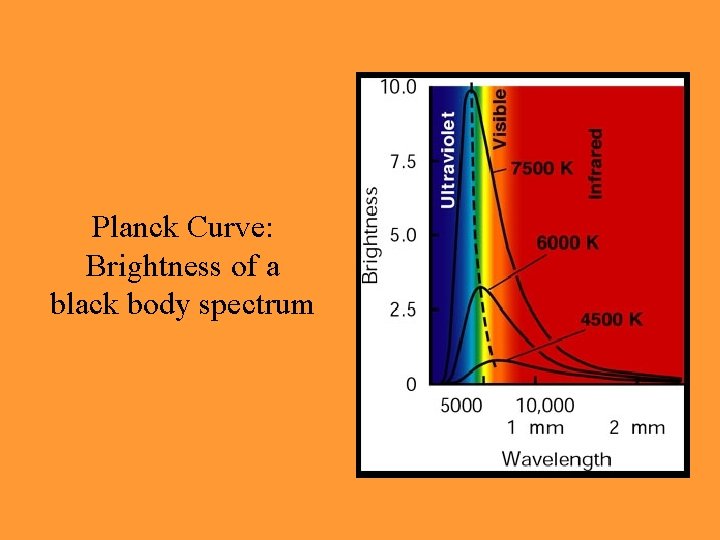
How Light is Emitted: ‘Black Body’ Radiation • Ideal object that gives off radiation • Perfectly absorbs all radiation, then re-emits radiation depending on temperature • Hot object appears ‘bluer’, cold object appears ‘redder’

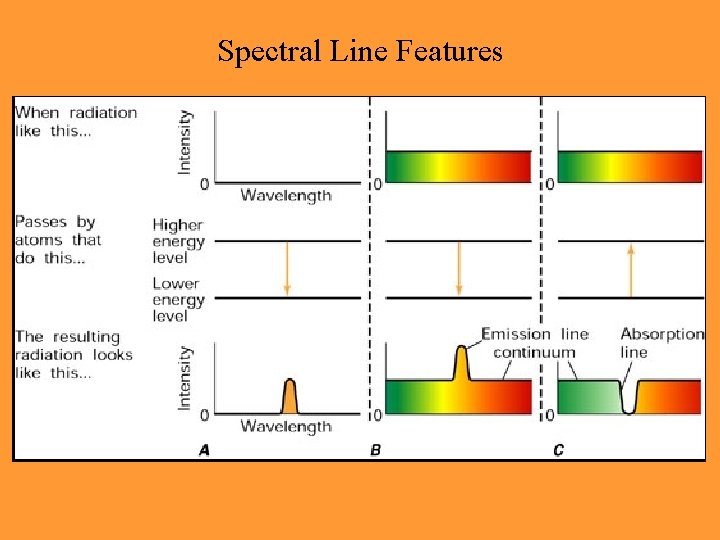
Observed Spectra • Absorption lines: occur when a cool gas lies in the line-of-sight between a hot object and the observer • Emission lines: occur in hot gases (a cooling mechanism), best seen toward dark background

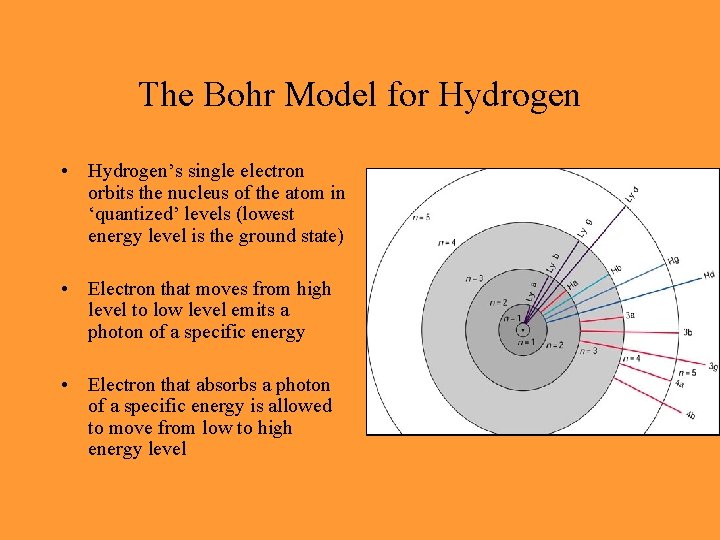
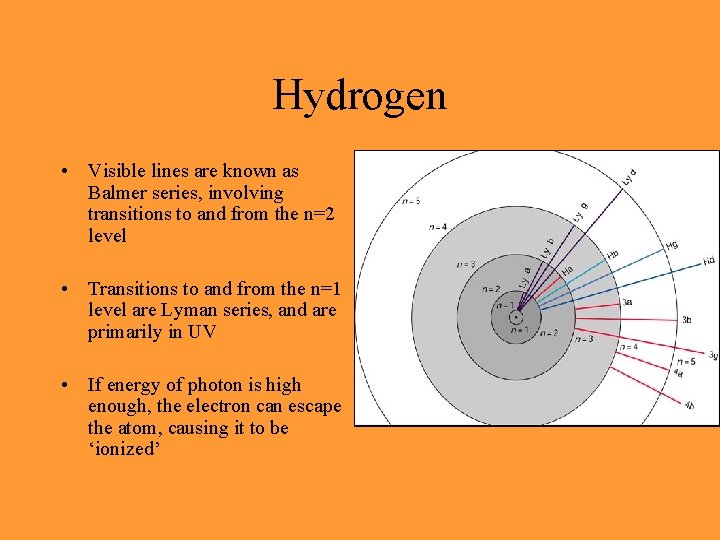
The Bohr Model for Hydrogen • Hydrogen’s single electron orbits the nucleus of the atom in ‘quantized’ levels (lowest energy level is the ground state) • Electron that moves from high level to low level emits a photon of a specific energy • Electron that absorbs a photon of a specific energy is allowed to move from low to high energy level

Planck Curve: Brightness of a black body spectrum

• Wavelength of spectrum’s peak found from Wien’s law T = 0. 29 cm·K • Integrated brightness emitted found from Stephan-Boltzmann law E = T 4 (energy per area per time)

• Wavelength of spectrum’s peak found from Wien’s law T = 0. 29 cm·K • Integrated brightness emitted found from Stephan-Boltzmann law E = T 4 (energy per area per time)

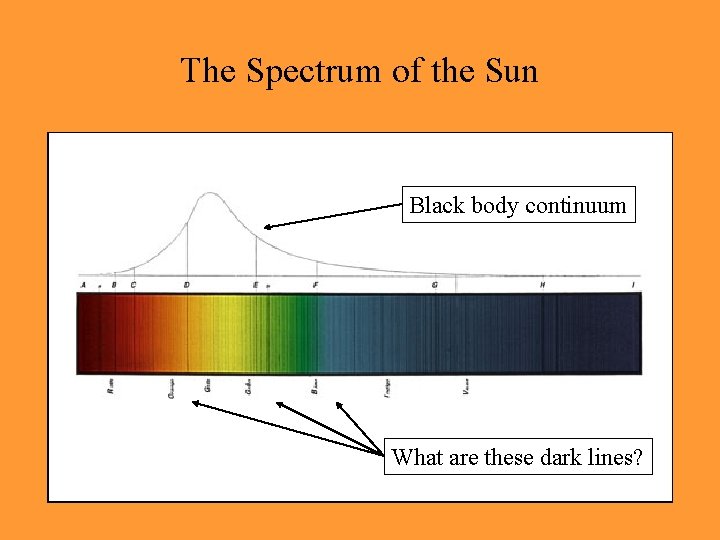
The Spectrum of the Sun Black body continuum What are these dark lines?

• Fraunhofer lines in the Solar spectrum – absorption of specific wavelengths by cool gas in front of a black body radiator

Atomic Radiation and Absorption: Spectral Lines • Atoms absorb and emit wavelengths of light specific to each chemical element • This evidence is the basis formation of quantum theory • Electrons in atoms absorb or emit photons of light of a particular wavelength, and change their orbital energy level

Spectral Line Features

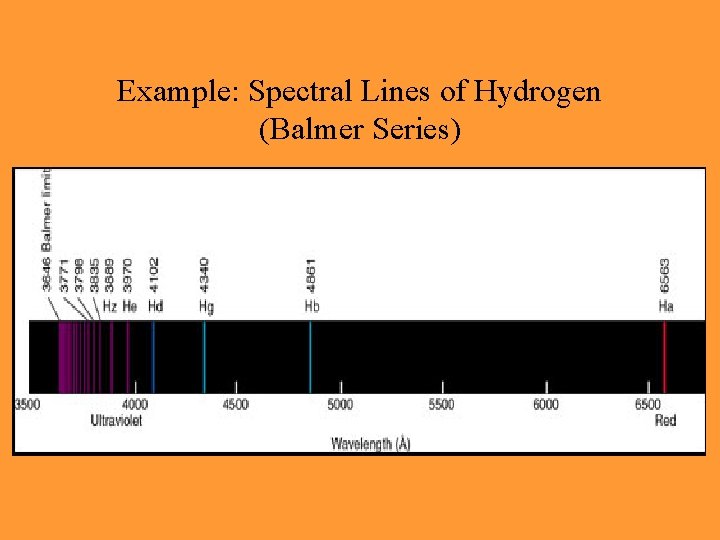
Example: Spectral Lines of Hydrogen (Balmer Series)

Hydrogen • Visible lines are known as Balmer series, involving transitions to and from the n=2 level • Transitions to and from the n=1 level are Lyman series, and are primarily in UV • If energy of photon is high enough, the electron can escape the atom, causing it to be ‘ionized’