Light and Quantized Energy 1 2 Electromagnetic Radiation




- Slides: 16

Light and Quantized Energy 1

2 Electromagnetic Radiation • Electromagnetic radiation: A form of energy that exhibits wavelike behavior as it travels through space • Light: a form of electromagnetic radiation

3 What are some examples of electromagnetic radiation? What else?

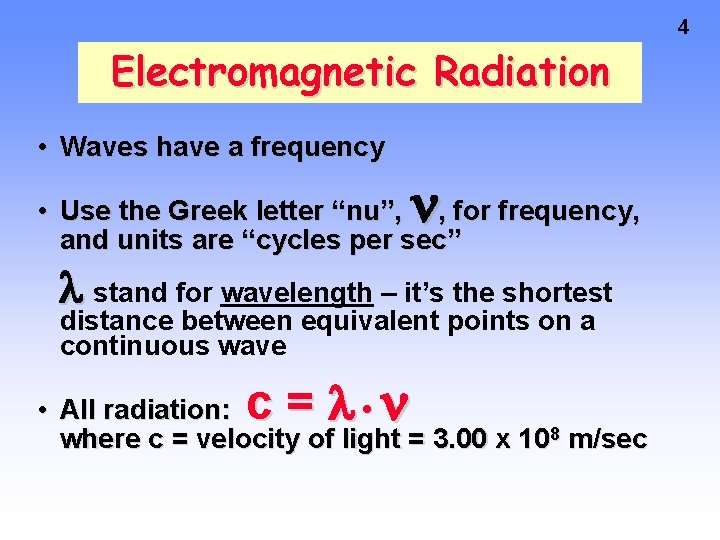
4 Electromagnetic Radiation • Waves have a frequency • Use the Greek letter “nu”, , for frequency, and units are “cycles per sec” stand for wavelength – it’s the shortest distance between equivalent points on a continuous wave c= • All radiation: • where c = velocity of light = 3. 00 x 108 m/sec

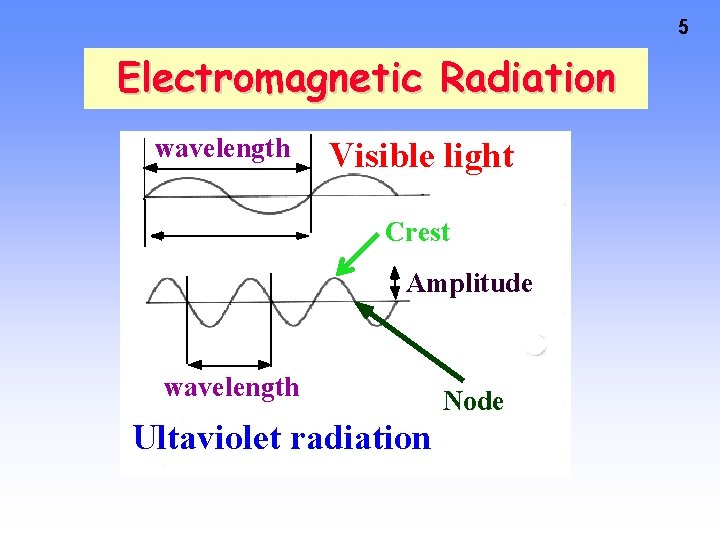
5 Electromagnetic Radiation wavelength Visible light Crest Amplitude wavelength Ultaviolet radiation Node

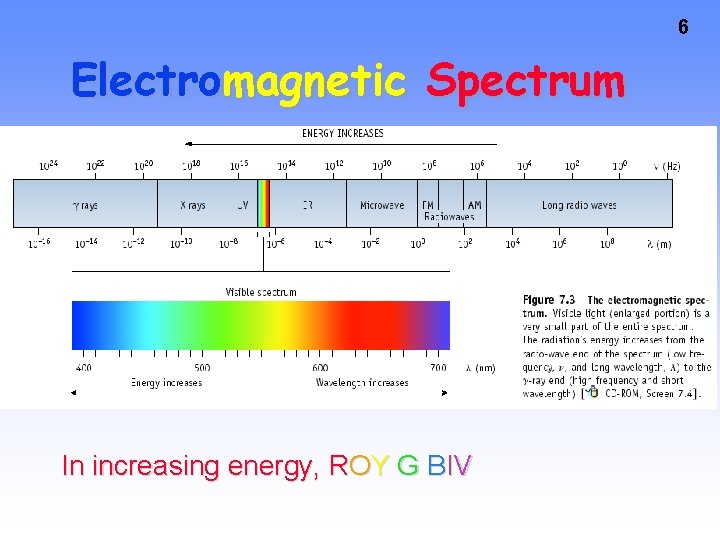
6 Electromagnetic Spectrum In increasing energy, ROY G BIV

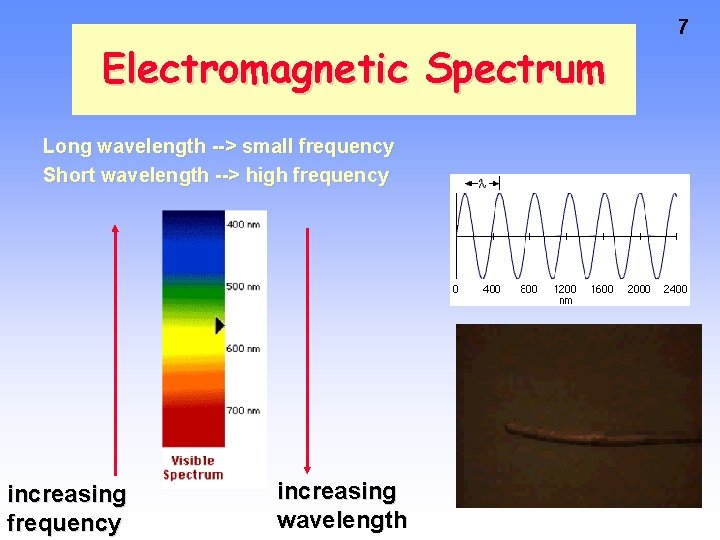
Electromagnetic Spectrum Long wavelength --> small frequency Short wavelength --> high frequency increasing wavelength 7

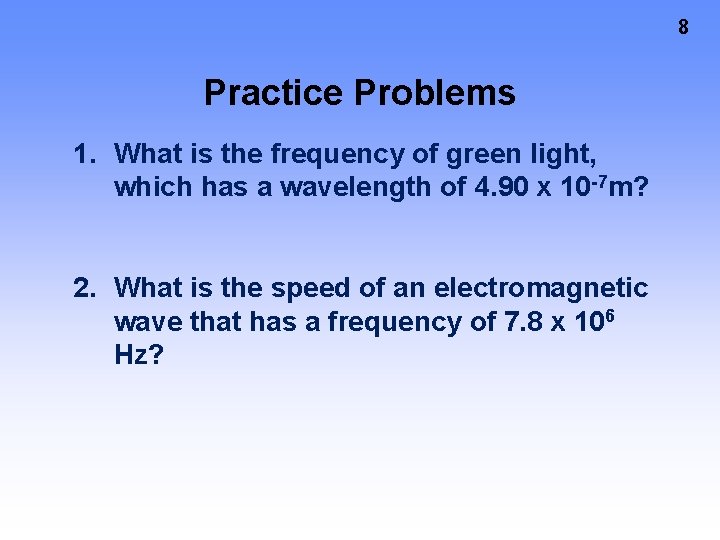
8 Practice Problems 1. What is the frequency of green light, which has a wavelength of 4. 90 x 10 -7 m? 2. What is the speed of an electromagnetic wave that has a frequency of 7. 8 x 106 Hz?

9 Quantum Concept • 1900 German physicist Max Planck started to search for WHY light emitted from heated objects. • Found that QUANTUM is the minimum amount of energy that can be gained or lost by an atom • Matter can gain or lose energy in small specific amounts called quanta

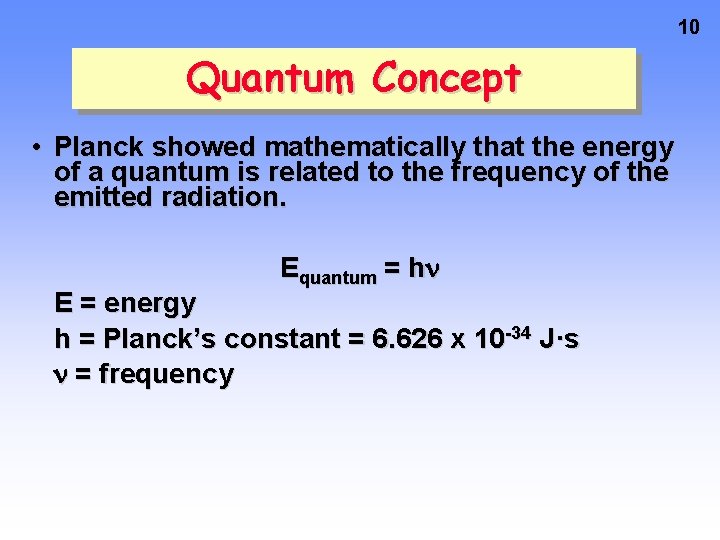
10 Quantum Concept • Planck showed mathematically that the energy of a quantum is related to the frequency of the emitted radiation. Equantum = h E = energy h = Planck’s constant = 6. 626 x 10 -34 J·s = frequency

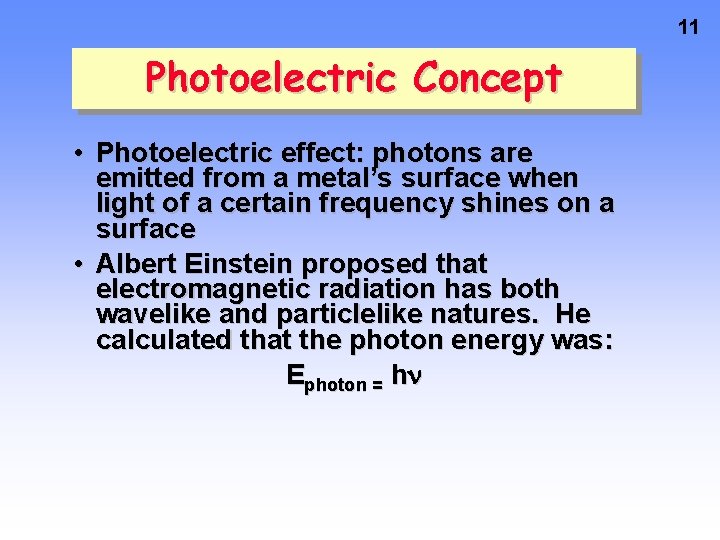
11 Photoelectric Concept • Photoelectric effect: photons are emitted from a metal’s surface when light of a certain frequency shines on a surface • Albert Einstein proposed that electromagnetic radiation has both wavelike and particlelike natures. He calculated that the photon energy was: Ephoton = h

Electromagnetic radiation. 12

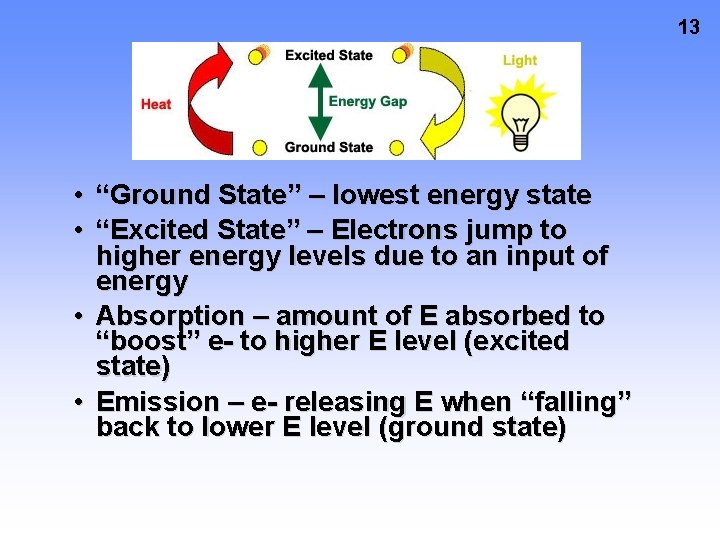
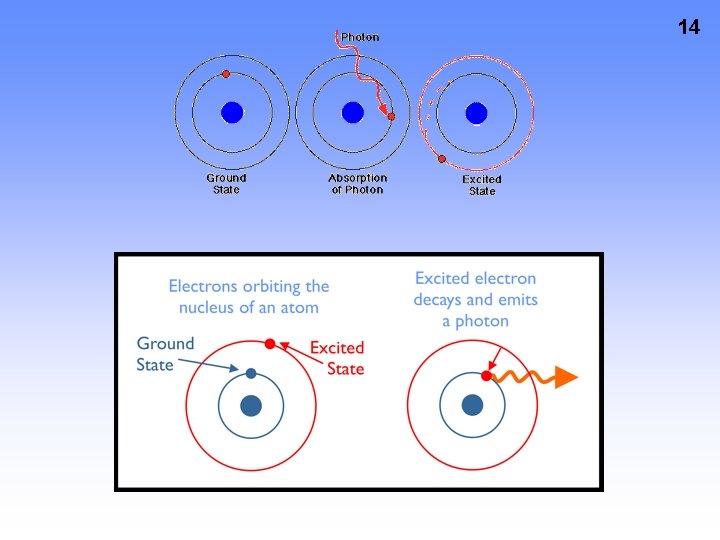
13 • “Ground State” – lowest energy state • “Excited State” – Electrons jump to higher energy levels due to an input of energy • Absorption – amount of E absorbed to “boost” e- to higher E level (excited state) • Emission – e- releasing E when “falling” back to lower E level (ground state)

14

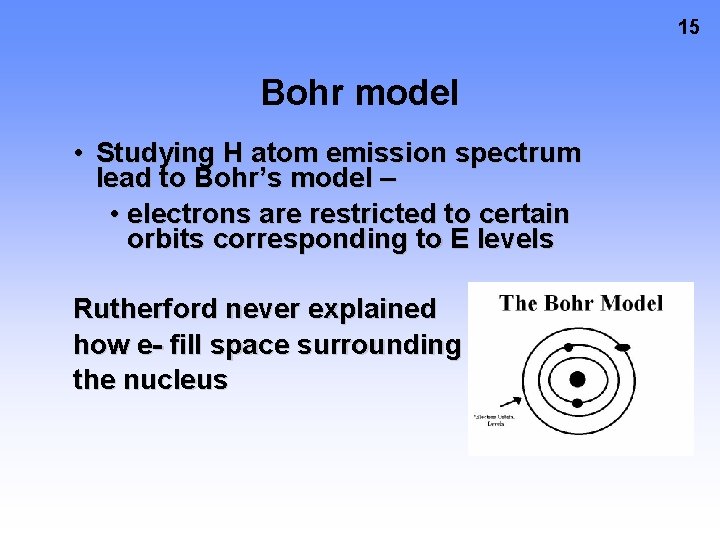
15 Bohr model • Studying H atom emission spectrum lead to Bohr’s model – • electrons are restricted to certain orbits corresponding to E levels Rutherford never explained how e- fill space surrounding the nucleus

16 Practice Problems 1. What is the energy for each of the following types of radiation? a. 6. 32 x 1020 s-1 b. 9. 50 x 1013 Hz c. 1. 05 x 1016 s-1