Light and Atomic Structure www assignmentpoint com Light
























- Slides: 25

Light and Atomic Structure www. assignmentpoint. com

Light and Atomic Structure • Light and its properties • Atomic structure • Interaction between Light and Matter • Spectrum www. assignmentpoint. com

Light in Everyday Life • Light is a form of energy, radiative energy 1 Watt = 1 Joule/sec • Light has color A prism split light into a spectrum (rainbow of colors) Light travels with a speed of c = 300, 000 km/s www. assignmentpoint. com

Rainbow www. assignmentpoint. com

Interaction of Light and Matter • Emission • Absorption • Transmission (passing through) • Reflection (scattering) www. assignmentpoint. com

Properties of Light behaves as both a particle and a wave Light particles are called photons, which can be counted individually. Light is also an electromagnetic wave The wavelength is the distance between adjacent peaks of the electric or magnetic field 1 nm (nanometer) = 10– 9 m 1μm (micron) = 10– 6 m The frequency is the number of peaks that pass by any point each second, measured in cycles per second or Hertz (Hz). light demo www. assignmentpoint. com

Light is an electromagnetic wave Light consists of many individual photons. Each travels at the speed c and can be www. assignmentpoint. comand a frequency. characterized by a wavelength

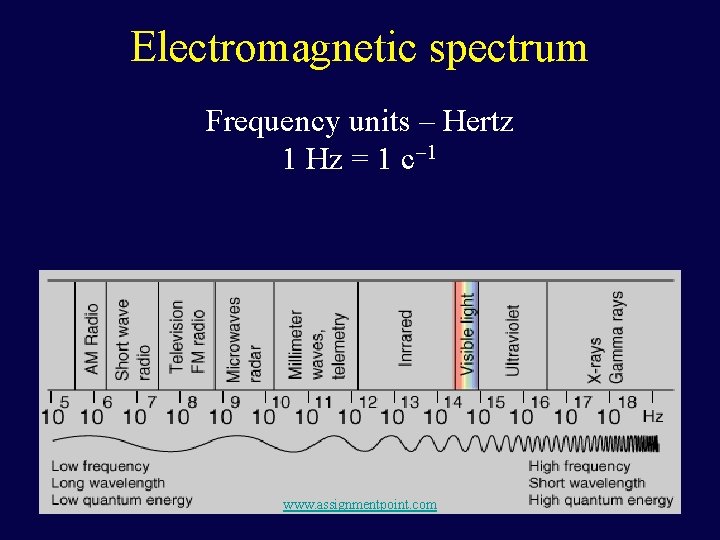
Many Forms of Light The spectrum of light is called the electromagnetic spectrum Different portions of the spectrum are called: The visible light - what we see with our eyes The infrared light - beyond of the red end of rainbow The ultraviolet light - beyond the blue end Radio waves - light with the longest wavelengths X rays - wavelengths shorter than ultraviolet Gamma rays - the shortest wavelength light www. assignmentpoint. com

Electromagnetic spectrum www. assignmentpoint. com

Electromagnetic spectrum Frequency units – Hertz 1 Hz = 1 c 1 www. assignmentpoint. com

Light and Matter The amount of light is called intensity Studying spectra of celestial bodies one can learn a wealth of information about them www. assignmentpoint. com

Atomic Structure 92 chemical elements have been identified in the Universe. Nearly 20 more have been created artificially. Each chemical element is made from a different type of atom. Atoms are made from particles called protons, neutrons, and electrons. Protons and neutrons form the nucleus in the center of the atom. Electrons surround the nucleus. www. assignmentpoint. com

Atomic Structure Positively charged protons are hold together by the strong force, which overcomes electrical repulsion. Negatively charged electrons are attracted to the nucleus. The number of protons in an atom is called the atomic number, which is unique for different chemical elements. The combined number of protons and neutrons in an atom is called the atomic mass number. Atoms of the same element with different number of www. assignmentpoint. com neutrons are called isotopes.

Absorption and Emission in Gases Since electrons in atoms can have only specific energies, the atoms can absorb or release energy only in these amounts (quanta) Electron gets energy, jumps to an excited state, release the energy, and falls back down The energy is emitted as a photon of light The photon has exactly the same energy that the electron has www. assignmentpoint. com lost

Types of Spectra Emission line spectrum consists of photons emitted as each electron falls back to lower levels Absorption line spectrum appears when photons are absorbed, causing electrons to jump up in energy Each element or molecule produces its own distinct set of spectral lines www. assignmentpoint. com

Emission by Hydrogen www. assignmentpoint. com

Hydrogen lines in the visible www. assignmentpoint. com

Examples of Spectra www. assignmentpoint. com

Thermal Radiation ``Complex’’ objects - planets, stars, people produce thermal radiation Its spectrum depends only on the object’s temperature Hotter objects emit more total radiation per unit surface area The radiated energy is proportional to the fourth power of the temperature Hotter objects emit photons with a higher average energy www. assignmentpoint. com

Temperature and Color www. assignmentpoint. com

Temperature and Intensity www. assignmentpoint. com

Reflected light When the light (for example, sunlight) strikes an object (ground, clouds, people), we see only the wavelengths of light that are reflected Different objects (fruits, rocks, atmospheric gases) reflect and absorb light at different wavelengths www. assignmentpoint. com

The Doppler Shift Radial motion of a distant object can be determined due to the Doppler effect The Doppler effect causes shifts in the wavelengths of light If an object is moving toward us, its entire spectrum is shifted to shorter wavelengths Because shorter wavelengths of the visible light are bluer, the Doppler shift of this object is called a blueshift The Doppler shift ofwww. assignmentpoint. com a moving away object - redshift

Doppler Effect Demo Doppler effect www. assignmentpoint. com

Summary Spectral information gives us more knowledge about the objects (composition, surface temperature, moving properties) Visible light is only a small portion of the electromagnetic spectrum The Doppler effect tells us how quickly light is moving toward or away from us www. assignmentpoint. com