Life substances Objectives Classify the variety of organic

Life substances Objectives: Classify the variety of organic compounds. Describe how polymers are formed and broken down in organisms. Compare the chemical structures of carbohydrates, lipids, proteins, and nucleic acids, and relate their importance to living things. Identify the effects of enzymes.
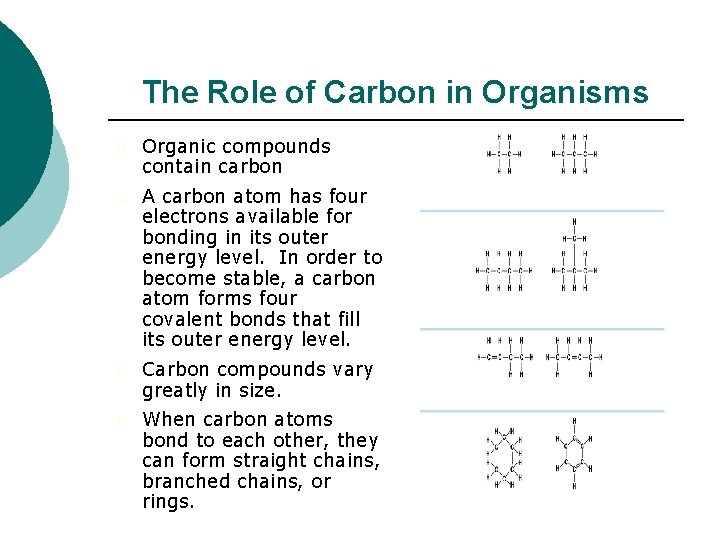
The Role of Carbon in Organisms ¡ Organic compounds contain carbon ¡ A carbon atom has four electrons available for bonding in its outer energy level. In order to become stable, a carbon atom forms four covalent bonds that fill its outer energy level. ¡ Carbon compounds vary greatly in size. ¡ When carbon atoms bond to each other, they can form straight chains, branched chains, or rings.
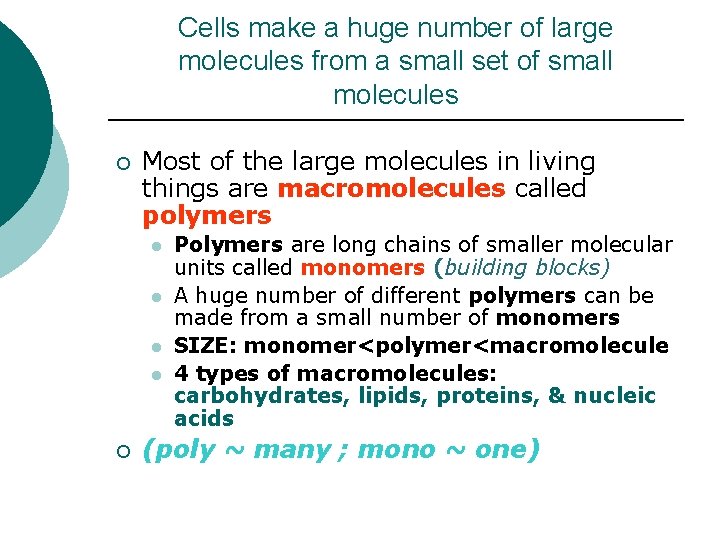
Cells make a huge number of large molecules from a small set of small molecules ¡ Most of the large molecules in living things are macromolecules called polymers l l ¡ Polymers are long chains of smaller molecular units called monomers (building blocks) A huge number of different polymers can be made from a small number of monomers SIZE: monomer<polymer<macromolecule 4 types of macromolecules: carbohydrates, lipids, proteins, & nucleic acids (poly ~ many ; mono ~ one)
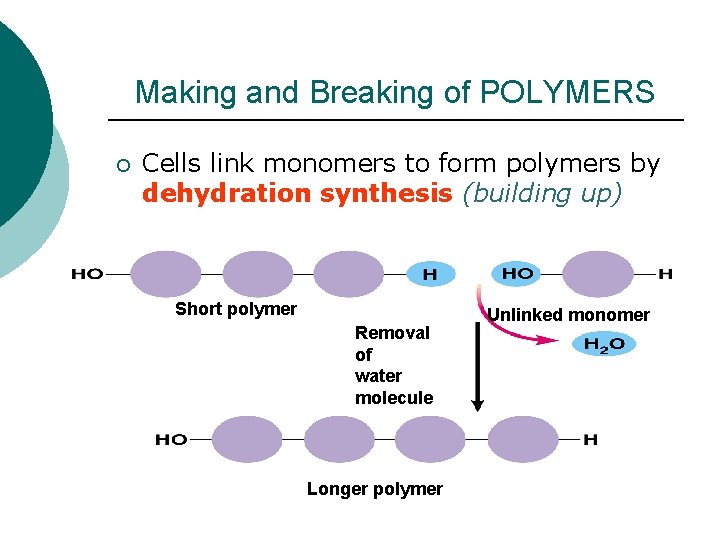
Making and Breaking of POLYMERS ¡ Cells link monomers to form polymers by dehydration synthesis (building up) Short polymer Removal of water molecule Longer polymer Unlinked monomer
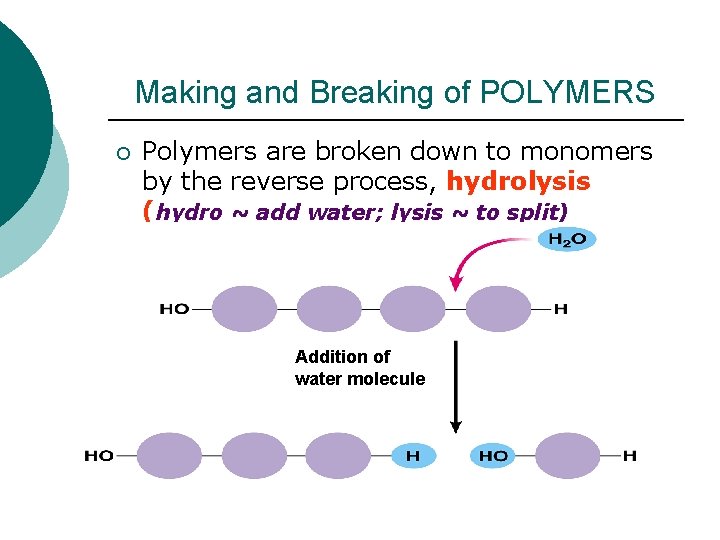
Making and Breaking of POLYMERS ¡ Polymers are broken down to monomers by the reverse process, hydrolysis (hydro ~ add water; lysis ~ to split) Addition of water molecule

1. CARBOHYDRATES ¡ composed of carbon, hydrogen, and oxygen with a ratio of about two hydrogen atoms and one oxygen atom for every carbon atom.
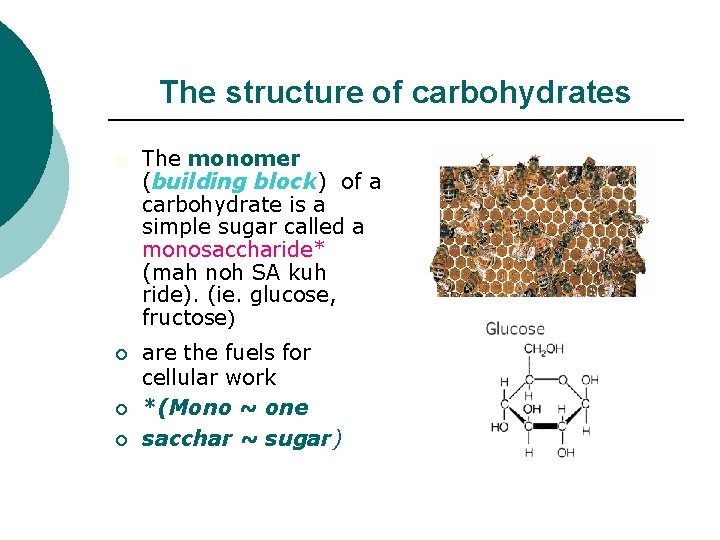
The structure of carbohydrates ¡ The monomer (building block) of a carbohydrate is a simple sugar called a monosaccharide* (mah noh SA kuh ride). (ie. glucose, fructose) ¡ are the fuels for cellular work *(Mono ~ one sacchar ~ sugar) ¡ ¡
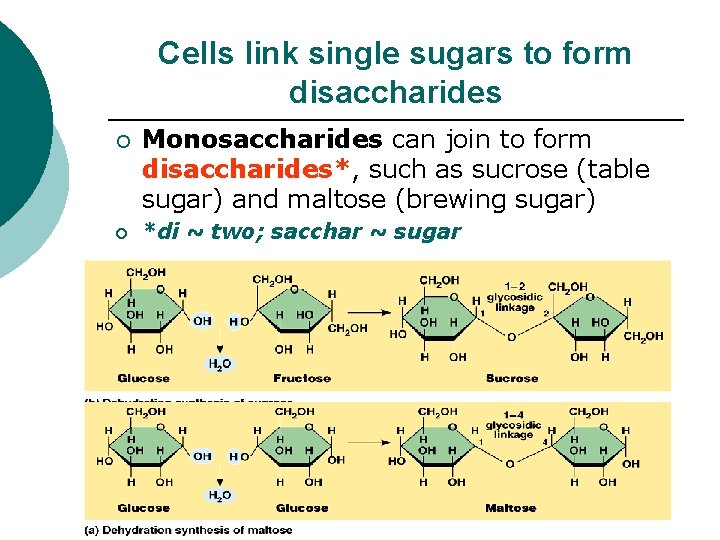
Cells link single sugars to form disaccharides ¡ ¡ Monosaccharides can join to form disaccharides*, such as sucrose (table sugar) and maltose (brewing sugar) *di ~ two; sacchar ~ sugar
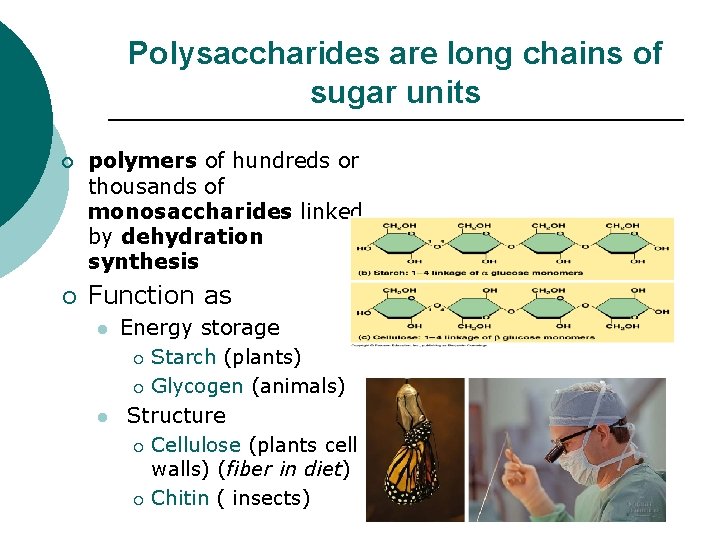
Polysaccharides are long chains of sugar units ¡ ¡ polymers of hundreds or thousands of monosaccharides linked by dehydration synthesis Function as l l Energy storage ¡ Starch (plants) ¡ Glycogen (animals) Structure ¡ Cellulose (plants cell walls) (fiber in diet) ¡ Chitin ( insects)

Lipids ¡ composed largely of carbon and hydrogen l l ¡ They are not true polymers They are grouped together because they do not mix with water (Nonpolar) (ie. fats, oils, waxes)
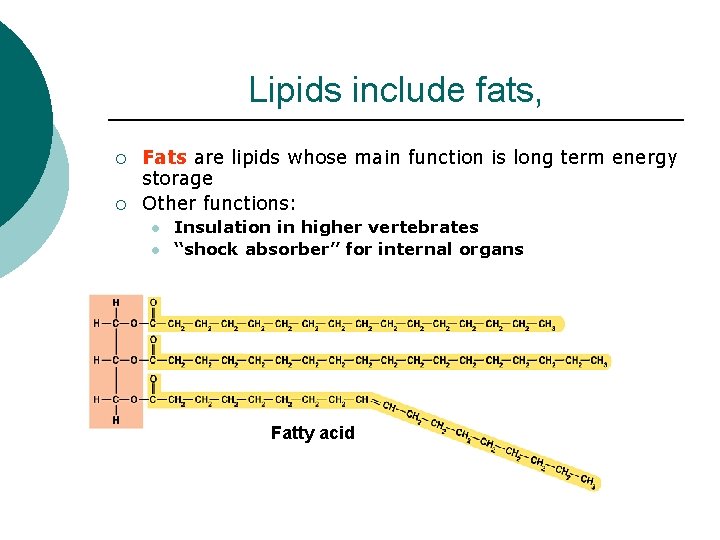
Lipids include fats, ¡ ¡ Fats are lipids whose main function is long term energy storage Other functions: l l Insulation in higher vertebrates “shock absorber” for internal organs Fatty acid
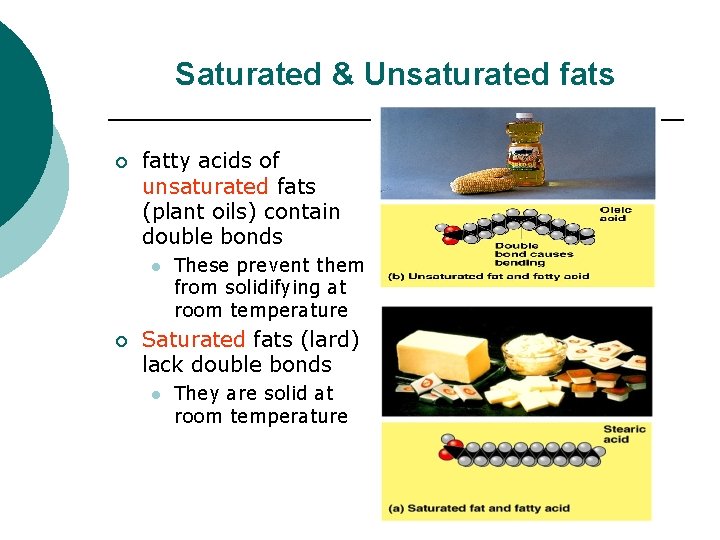
Saturated & Unsaturated fats ¡ fatty acids of unsaturated fats (plant oils) contain double bonds l ¡ These prevent them from solidifying at room temperature Saturated fats (lard) lack double bonds l They are solid at room temperature
PROTEINS ¡ ¡ essential to the structures and activities of life Make up 50% of dry weight of cells Contain carbon, hydrogen, & oxygen PLUS nitrogen and sometimes sulfur Proteins are involved in l l l ¡ cellular structure Movement (muscles) Defense (antibodies) Transport (blood) Communication Monomers are called amino acids
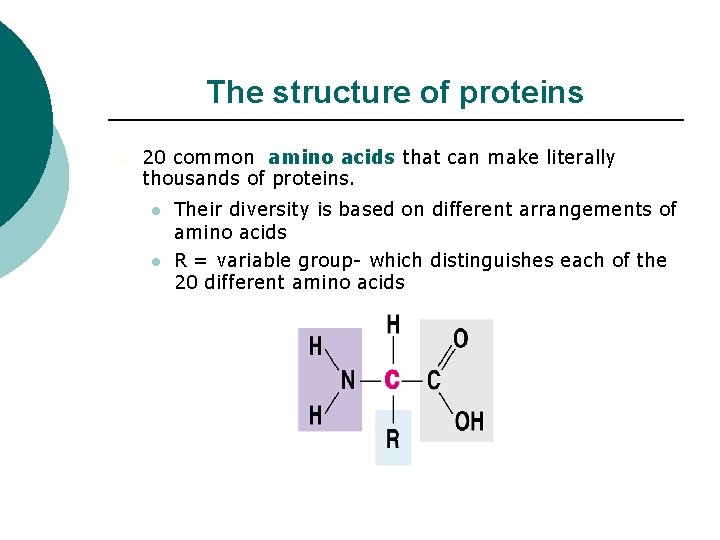
The structure of proteins ¡ 20 common amino acids that can make literally thousands of proteins. l l Their diversity is based on different arrangements of amino acids R = variable group- which distinguishes each of the 20 different amino acids
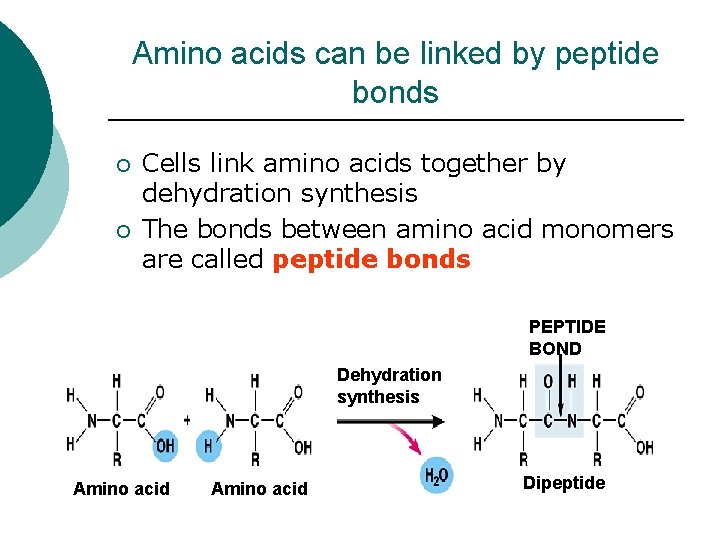
Amino acids can be linked by peptide bonds ¡ ¡ Cells link amino acids together by dehydration synthesis The bonds between amino acid monomers are called peptide bonds PEPTIDE BOND Dehydration synthesis Amino acid Dipeptide
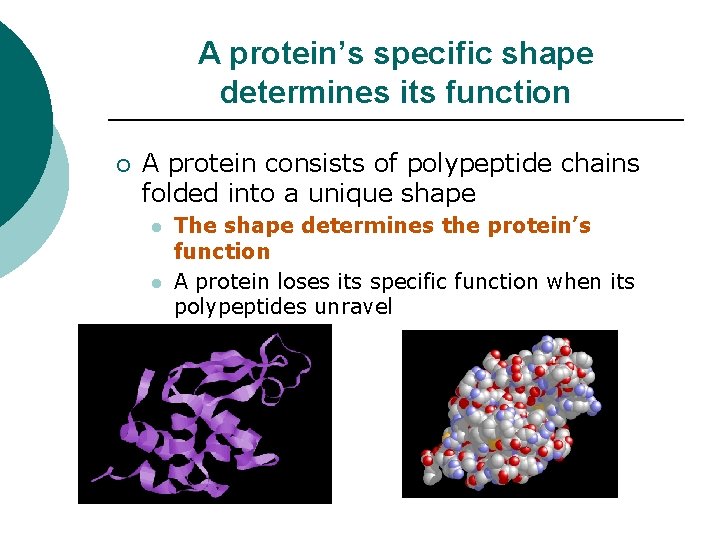
A protein’s specific shape determines its function ¡ A protein consists of polypeptide chains folded into a unique shape l l The shape determines the protein’s function A protein loses its specific function when its polypeptides unravel

Enzymes ¡ ¡ Enzymes are important proteins found in living things. An enzyme is a protein that speeds up the rate of a chemical reaction. (SEE SEPARATE LECTURE. )
Nucleic acids ¡ A nucleic (noo KLAY ihk) acid is a complex biomolecule that stores cellular information in the form of a code. ¡ 1. DNA (deoxyribonucleic acid) contains ¡ 2. RNA (ribonucleic acid) forms a copy of ¡ They ultimately control the life of a cell the instructions used to form all of an organism’s proteins. DNA for use in making proteins.
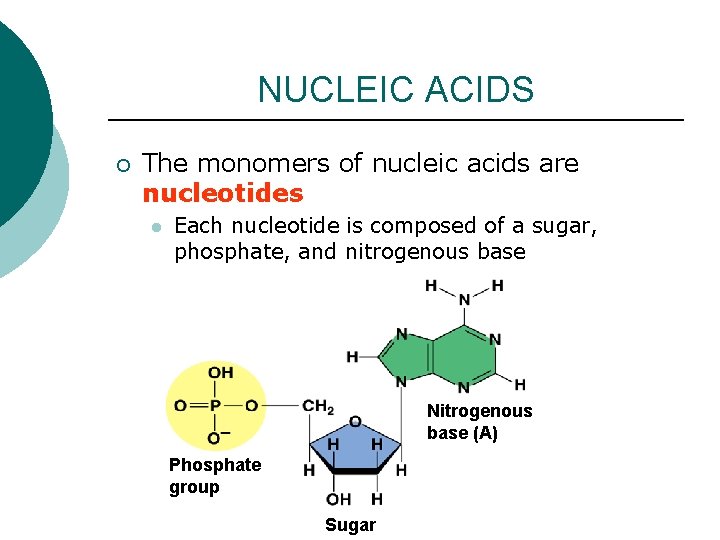
NUCLEIC ACIDS ¡ The monomers of nucleic acids are nucleotides l Each nucleotide is composed of a sugar, phosphate, and nitrogenous base Nitrogenous base (A) Phosphate group Sugar
- Slides: 19