LI Understand how IR Spectroscopy works Explain how






- Slides: 12

LI: Understand how IR Spectroscopy works Explain how infrared spectroscopy can be used to identify types of organic bonds Identify main peaks on an IR spectrum

Bonding recap – match the words to phrases Covalent bond A mathematical value for the centre of positive and negative charges in a molecule Polar bond Uneven sharing of electrons in a colvalent bond due to electronegativity differences Non- polar bond Electrons are shared between atoms and the nucleii move closer Molecular dipole A dipole that is induced by distortion of the electron cloud on a non-polar bond by a neighbouring molecule Instantaneous dipole Dipole moment When bond dipoles are assymetrically arranged around a central molecule Even sharing of electrons in a covalent bond

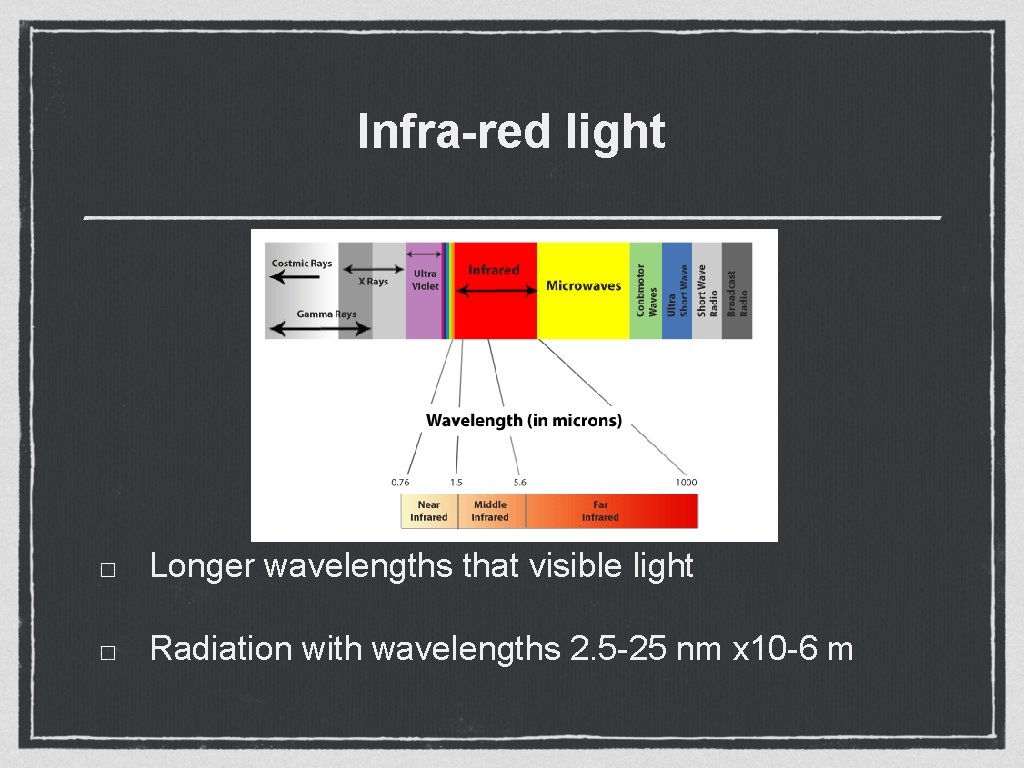
Infra-red light Longer wavelengths that visible light Radiation with wavelengths 2. 5 -25 nm x 10 -6 m

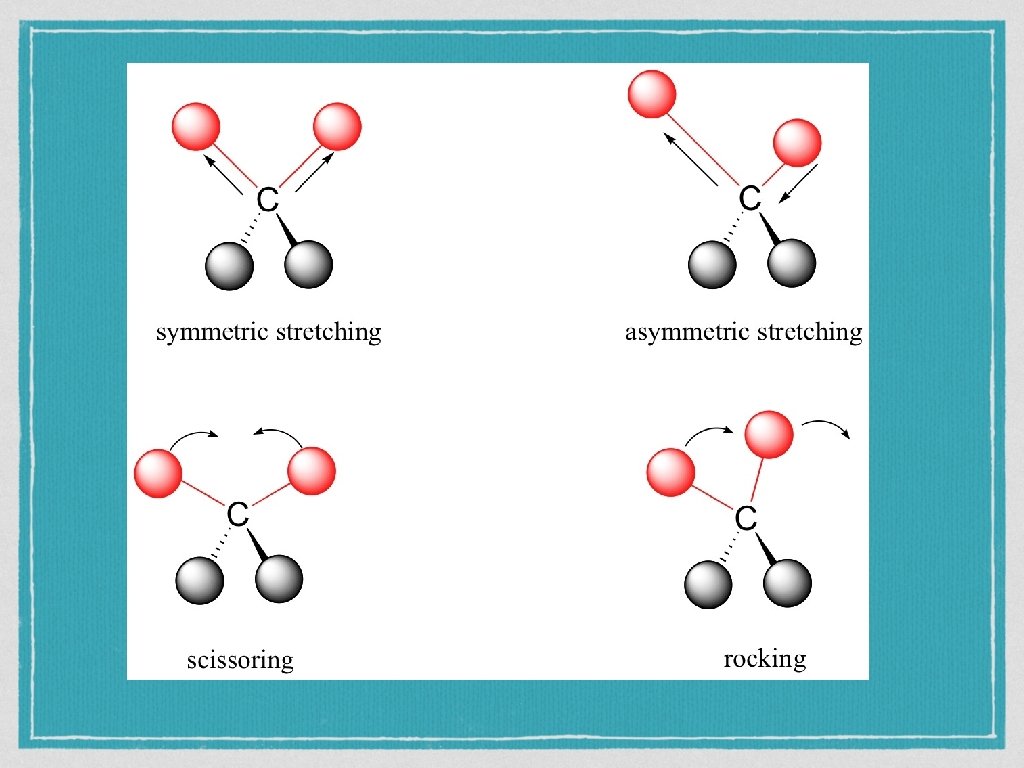
Covalent bonds are not really fixed in position as they are in our model kits They are more like springs between atoms and can be stretched in length and bent or twisted. http: //assign 3. chem. usyd. edu. au/Organic. Spectrosc opy/? type=Infrared

Infra-red light Radiation in the infrared (IR) region of the electromagnetic spectrum has the energy to excite vibrations of covalent bonds. The absorption of IR radiation causes bonds to stretch and bend. Stretches correspond to the increasing and decreasing of the bond lengths within a molecule. Bends correspond to the increasing and decreasing of the angle between bonds in a molecule.


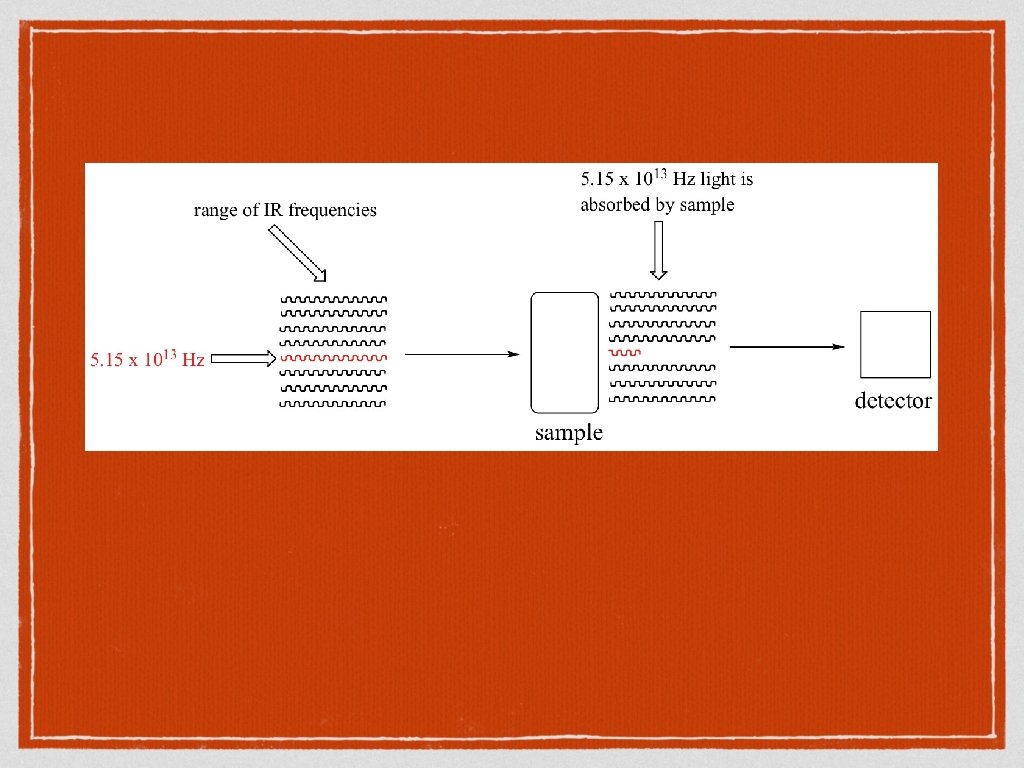
Creating the spectrum When IR radiation is shone on a molecule, it can only absorb it if: The energy of the radiation corresponds to the energy required to vibrate the molecule in a certain way, and The vibration leads to the dipole moment of the molecule changing. If radiation is absorbed at a particular wavelength, we see a peak on the spectrum.

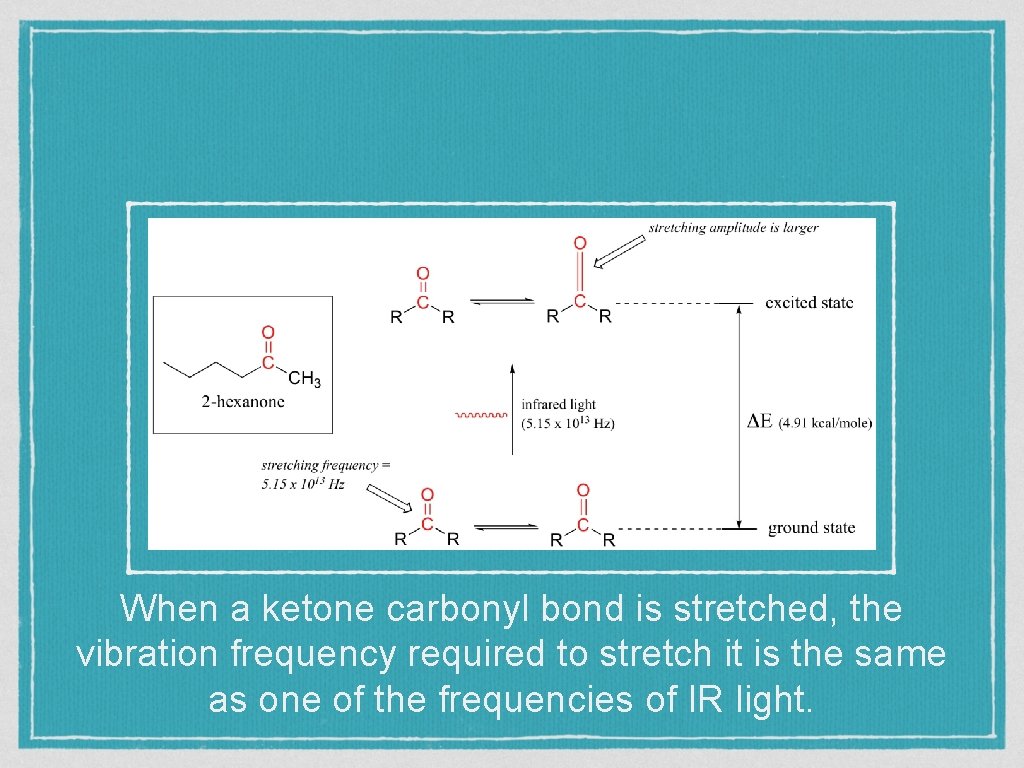
When a ketone carbonyl bond is stretched, the vibration frequency required to stretch it is the same as one of the frequencies of IR light.


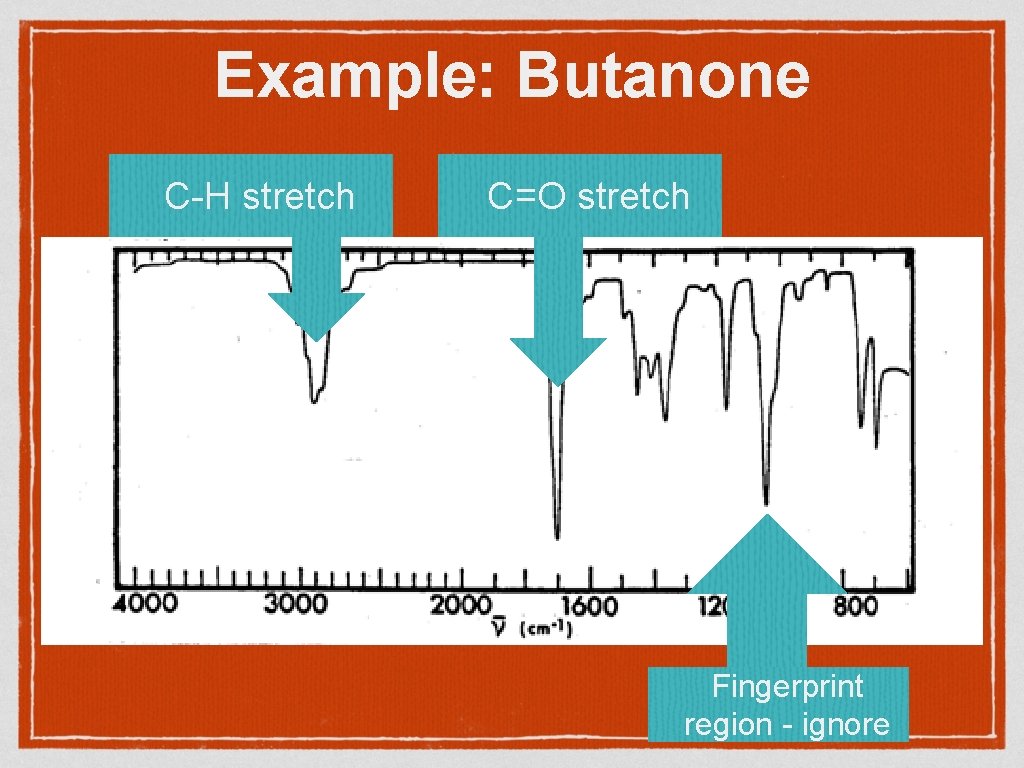
Wavenumber Infrared frequencies are recorded in wavenumber (cm -1) This number is inversely proprotional to wavelength (ie the bigger the wavenumber, the shorter and higher energy wavelength. Organic molecules have many bonds with many possible vibrations so infra – red spectra have many peaks. However some certain functional groups show at particular wavenumbers which can allow us to identify functional groups. Note that the spectrum is upside down as we are recording how much radiation is not transmitted through the sample (100% – absorption)

Example: Butanone C-H stretch C=O stretch Fingerprint region - ignore

Work time: Use page 12 of your workbooks to take notes on how infra red spectra are created and used to identify molecules. Then have a go at questions on worksheet. Your notes should include keywords: Wavenumber and % transmission Bond stretching and bond bending Dipole moment Vibrations Functional groups and fingerprint region