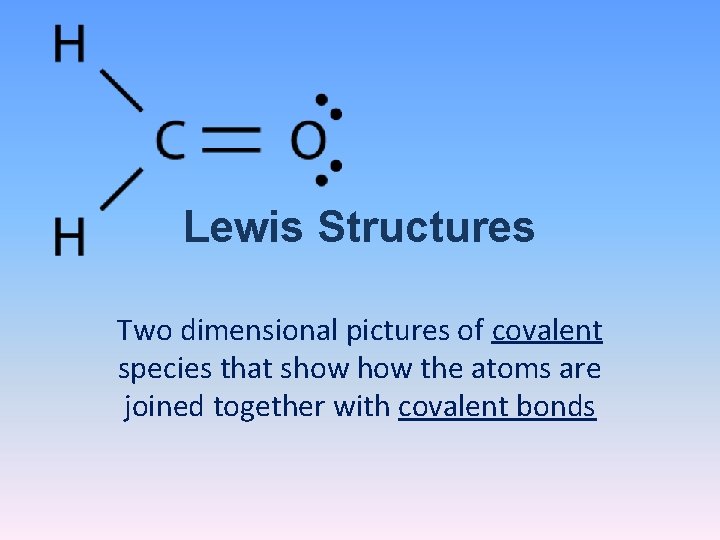
Lewis Structures Two dimensional pictures of covalent species




- Slides: 23

Lewis Structures Two dimensional pictures of covalent species that show the atoms are joined together with covalent bonds

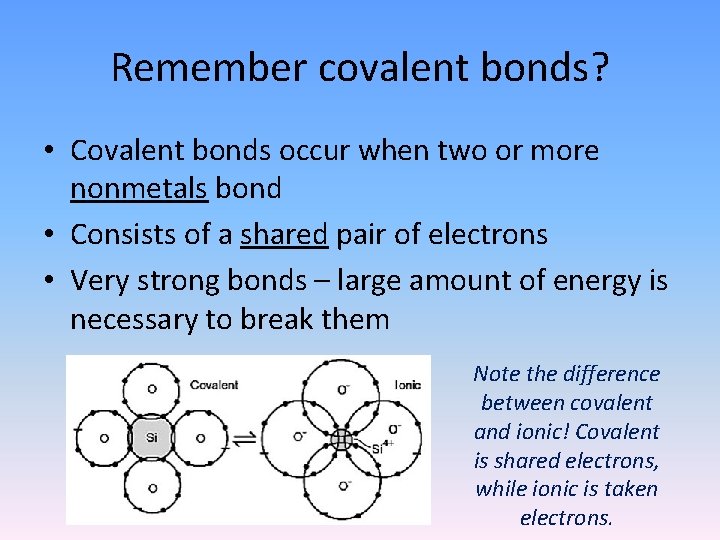
Remember covalent bonds? • Covalent bonds occur when two or more nonmetals bond • Consists of a shared pair of electrons • Very strong bonds – large amount of energy is necessary to break them Note the difference between covalent and ionic! Covalent is shared electrons, while ionic is taken electrons.

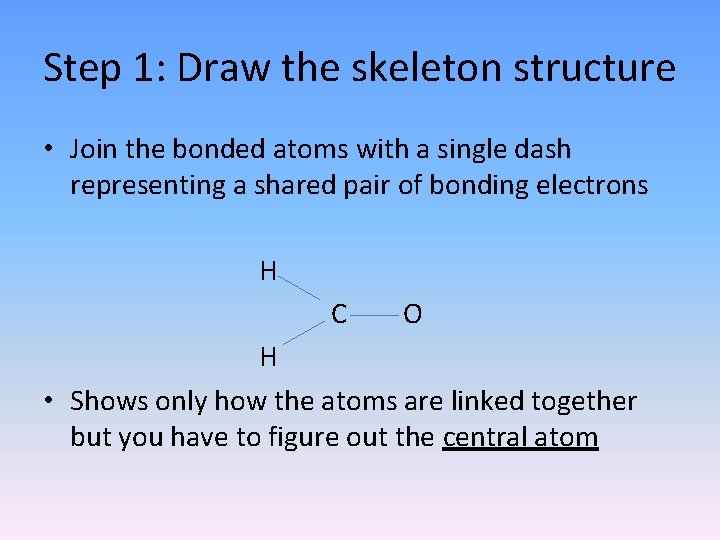
Step 1: Draw the skeleton structure • Join the bonded atoms with a single dash representing a shared pair of bonding electrons H C O H • Shows only how the atoms are linked together but you have to figure out the central atom

Step 1. 5: Identify the Central Atom 1. Neither hydrogen nor fluorine is ever a central atom. 2. The central atom usually appears only once in the formula. For example: PF 3, SO 42 -, CCl 4 3. If there are two atoms that could be the central atom, most often the atom of lower electronegativity will be the central atom

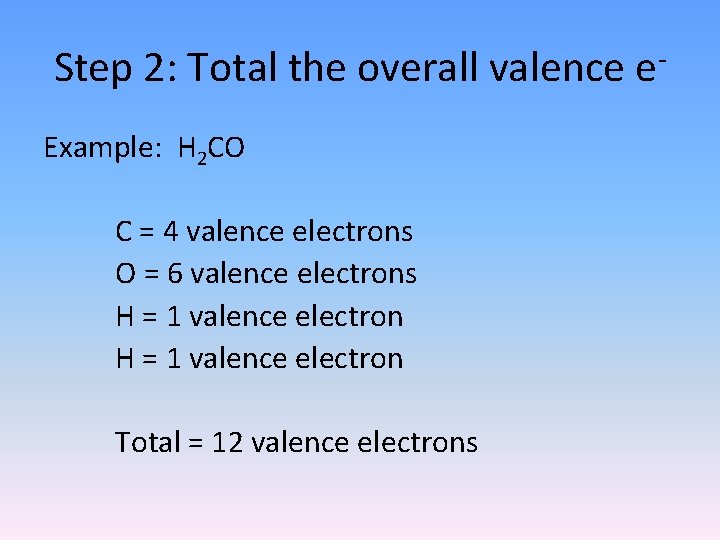
Step 2: Total the overall valence e. Example: H 2 CO C = 4 valence electrons O = 6 valence electrons H = 1 valence electron Total = 12 valence electrons

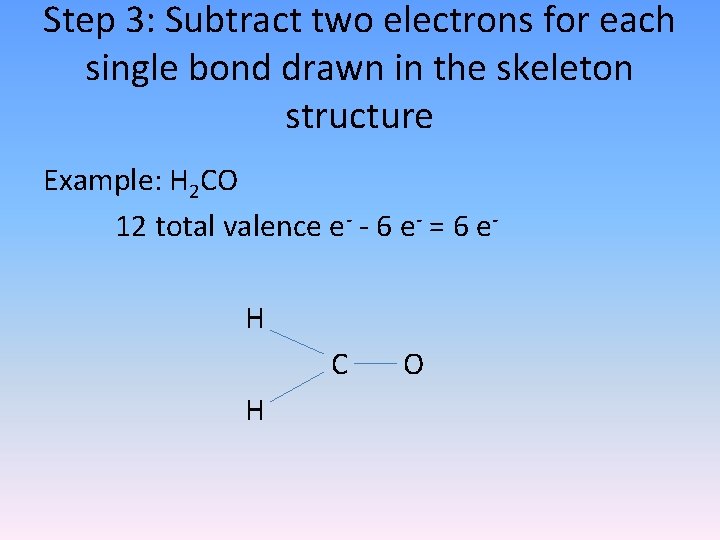
Step 3: Subtract two electrons for each single bond drawn in the skeleton structure Example: H 2 CO 12 total valence e- - 6 e- = 6 e. H C H O

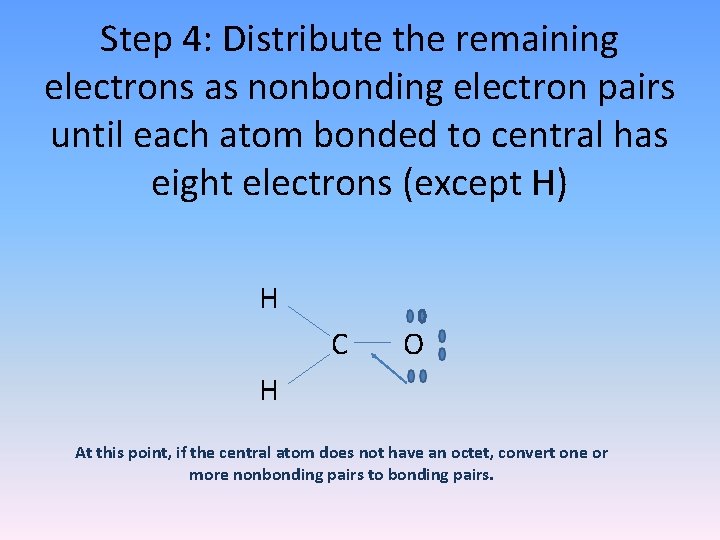
Step 4: Distribute the remaining electrons as nonbonding electron pairs until each atom bonded to central has eight electrons (except H) H C O H At this point, if the central atom does not have an octet, convert one or more nonbonding pairs to bonding pairs.

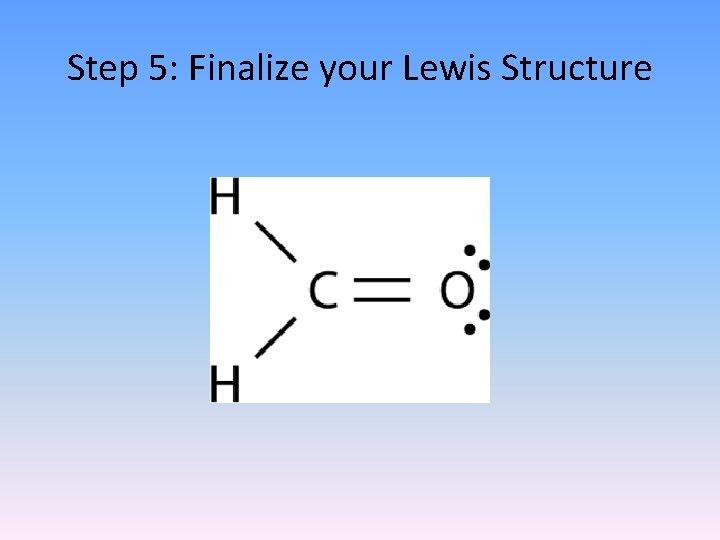
Step 5: Finalize your Lewis Structure

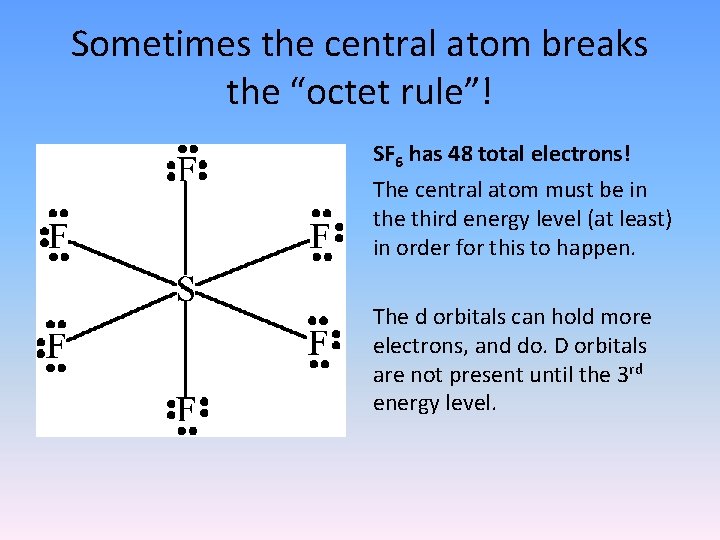
Sometimes the central atom breaks the “octet rule”! SF 6 has 48 total electrons! The central atom must be in the third energy level (at least) in order for this to happen. The d orbitals can hold more electrons, and do. D orbitals are not present until the 3 rd energy level.

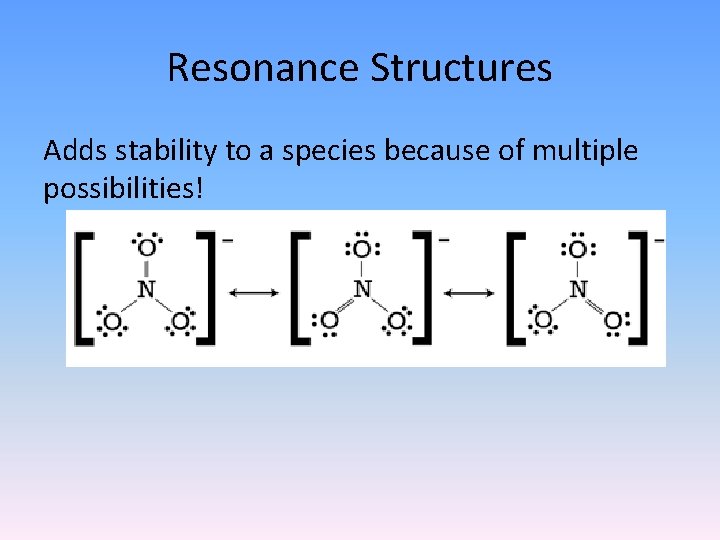
Resonance Structures Adds stability to a species because of multiple possibilities!

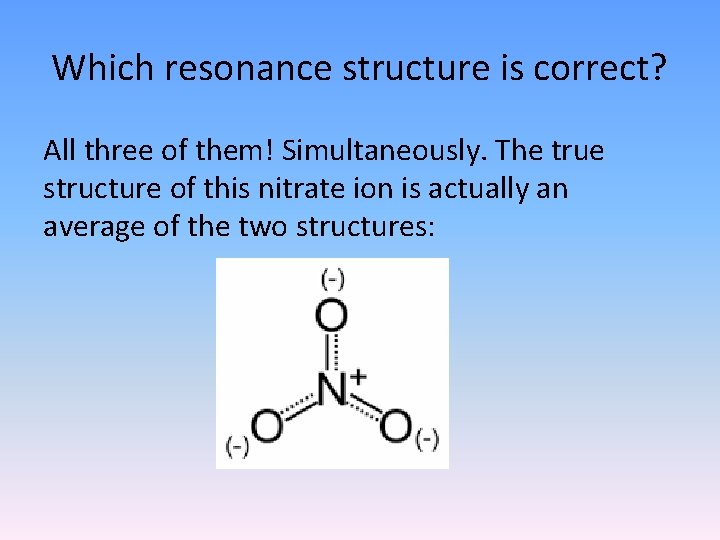
Which resonance structure is correct? All three of them! Simultaneously. The true structure of this nitrate ion is actually an average of the two structures:

Where will you observe resonance structures? 1. Polyatomic ions: not always, but it is NOT uncommon for PI to have resonance structures 2. Some organic compounds: not always, but very possible **Remember, only draw resonance structures when there’s more than one possible place the multiple bonds can be shown!

VSEPR Valence Shell Electron Pair Repulsion • Basically means that the outer electrons on an atom repel each other. • All e- have negative charge, this repulsion makes sense.

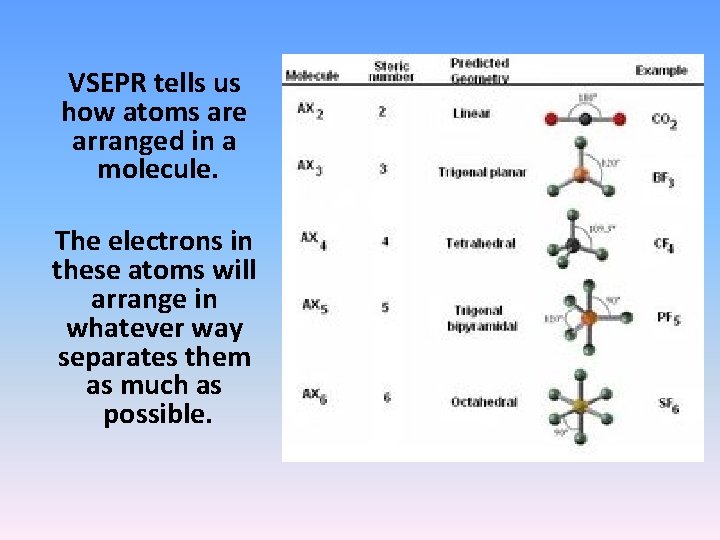
VSEPR tells us how atoms are arranged in a molecule. The electrons in these atoms will arrange in whatever way separates them as much as possible.

Hybridization The electron pairs on each atom all exist in something called hybrid orbitals. • Consist of some combination of the s-, p-, etc orbitals that allow the molecule to achieve angles keeping the atoms far apart from each other. s, sp, 2 sp , 3 sp d, 3 2 sp d

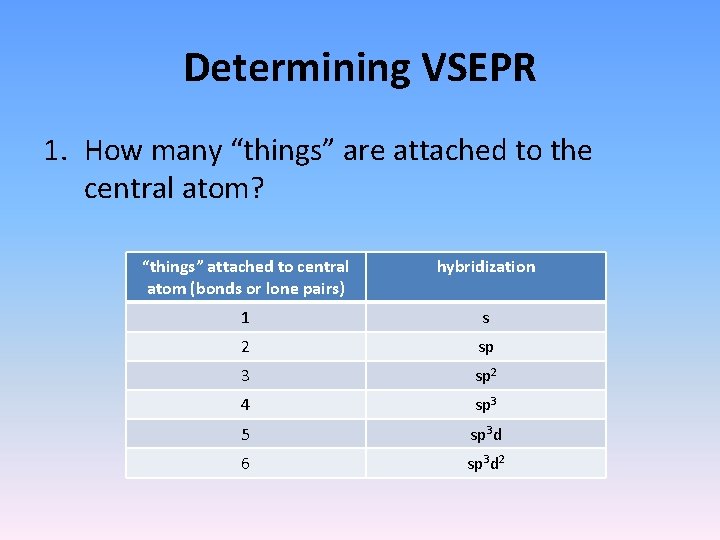
Determining VSEPR 1. How many “things” are attached to the central atom? “things” attached to central atom (bonds or lone pairs) hybridization 1 s 2 sp 3 sp 2 4 sp 3 5 sp 3 d 6 sp 3 d 2

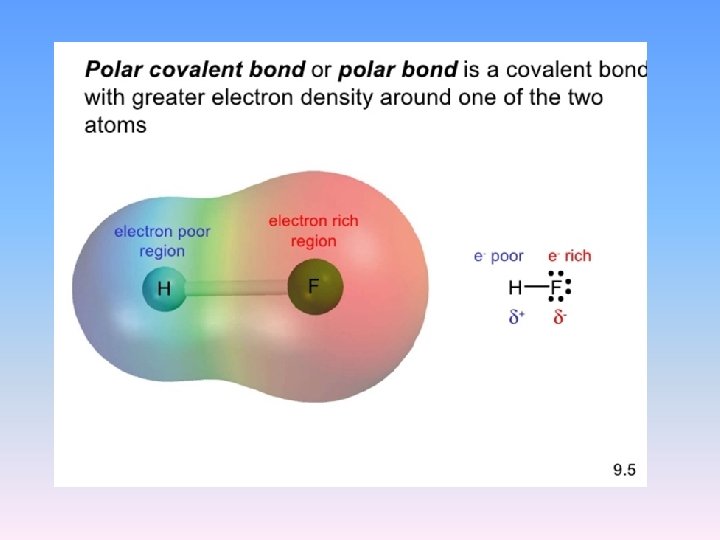
Polarity • Property of molecules that can be explained by their molecular shape • the atoms that make up a molecule have different electronegativities The result? Polar bonds – the electrons are shared unequally When there is this “unbalance” the molecule is having a dipole moment.


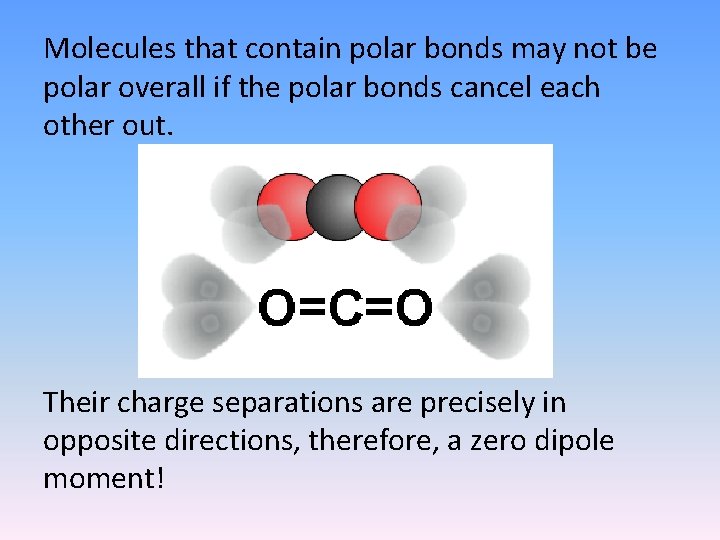
Molecules that contain polar bonds may not be polar overall if the polar bonds cancel each other out. Their charge separations are precisely in opposite directions, therefore, a zero dipole moment!

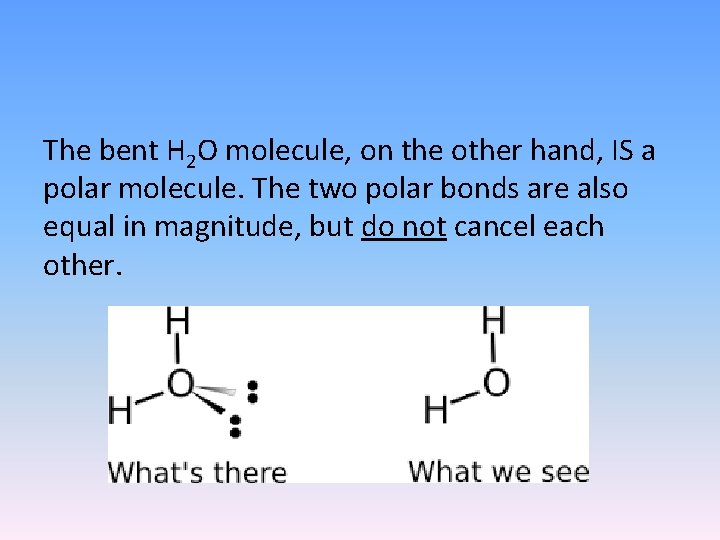
The bent H 2 O molecule, on the other hand, IS a polar molecule. The two polar bonds are also equal in magnitude, but do not cancel each other.


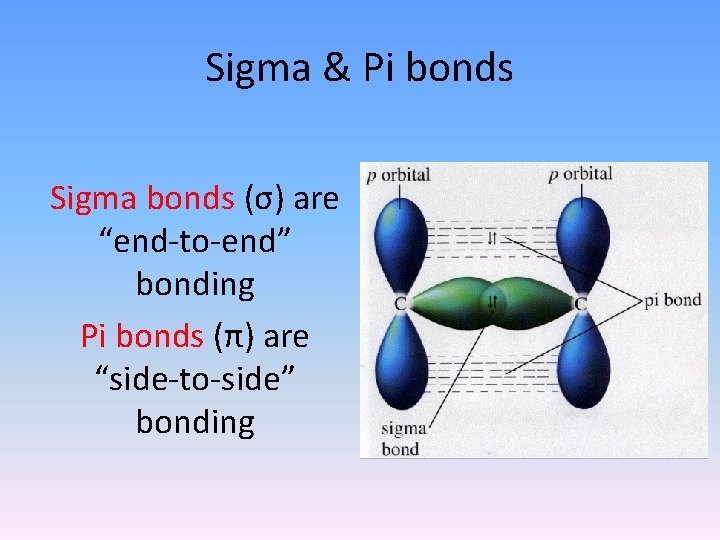
Sigma & Pi bonds Sigma bonds (σ) are “end-to-end” bonding Pi bonds (π) are “side-to-side” bonding

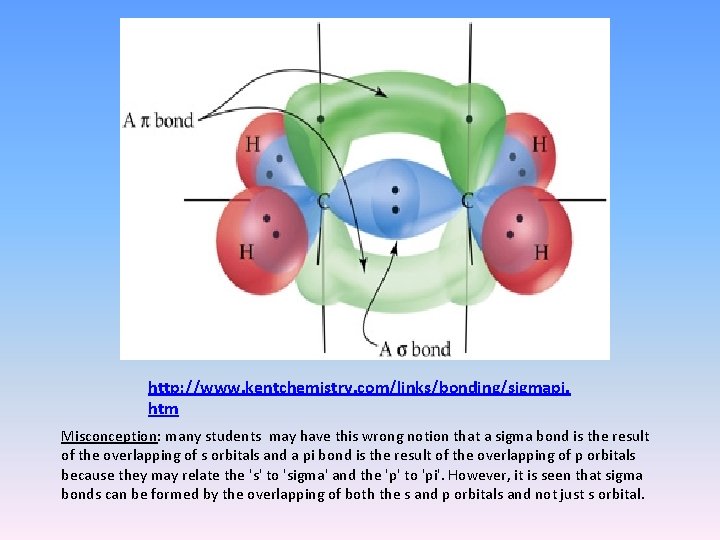
http: //www. kentchemistry. com/links/bonding/sigmapi. htm Misconception: many students may have this wrong notion that a sigma bond is the result of the overlapping of s orbitals and a pi bond is the result of the overlapping of p orbitals because they may relate the 's' to 'sigma' and the 'p' to 'pi'. However, it is seen that sigma bonds can be formed by the overlapping of both the s and p orbitals and not just s orbital.