Lewis structures and Binary ionic Compounds Ch 7

Lewis structures and Binary ionic Compounds Ch 7 P 194 -206

Objectives: At the end of this unit you will: Know and understand how to find the number of valence electrons in an atom Be able to create electron dot diagrams Explain how cations and anions are formed Write formulas and names for ionic compounds

Vocab: Valence electron Electron dot structure Ionic compound Ionic bond Chemical formula Formula unit Coordination number Metallic bond alloy

Review for a quick moment The absolute most important players in the game of bonding are the___ What causes the “attraction” b/w atoms in a bond? Define valence electrons

Review for a quick moment The absolute most important players in the game of bonding are the electrons What causes the “attraction” b/w atoms in a bond? The positive and negative charge Define valence e-: the electrons in the outermost energy level
More review for a quick moment The goal of every element on the PT is to _________ Elements achieve this goal by doing what?
More review for a quick moment The goal of every element on the PT is to become more like a noble gas Elements achieve this goal by doing what? Losing or gaining electrons
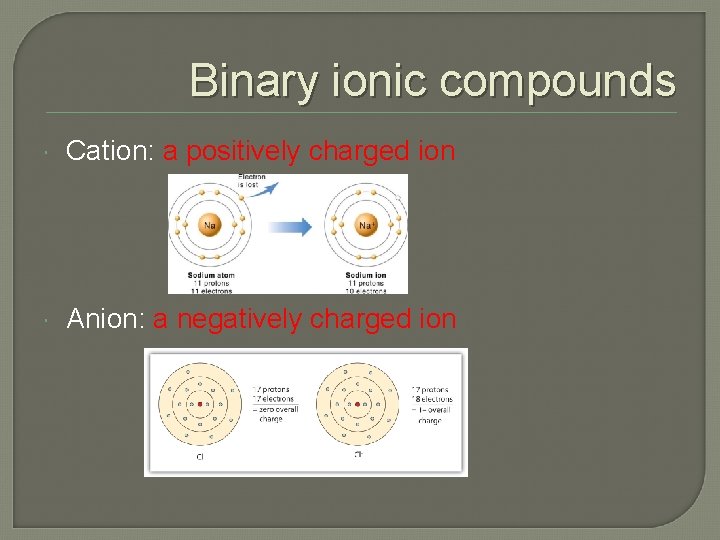
Binary ionic compounds Cation: a positively charged ion Anion: a negatively charged ion
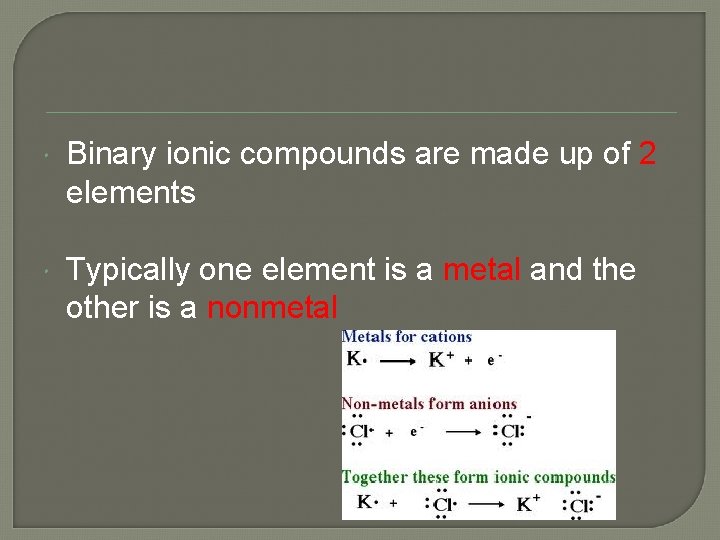
Binary ionic compounds are made up of 2 elements Typically one element is a metal and the other is a nonmetal

Binary ionic compounds Cations and anions have opposite charges and therefore attract one another. The force holding the charges together is called an ionic bond

Binary ionic Compounds An ionic bond is a transfer of electrons. We can represent this by drawing a lewis structure (dot diagram). Keeping in mind that the overall charge of the compound must be neutral
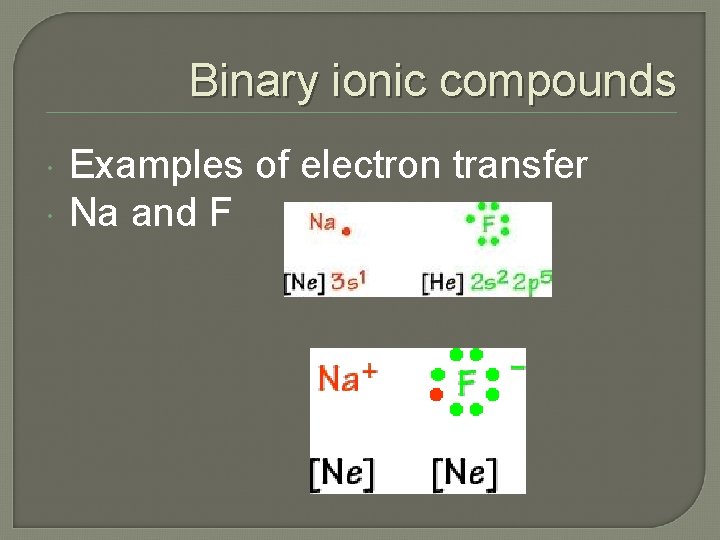
Binary ionic compounds Examples of electron transfer Na and F

Binary ionic compounds Examples of electron transfer Ca and Cl

Binary ionic compounds Try these: • K and I • Al and O

Naming rules 1. When naming, always place the cation name first and the anion name second. 2. The first element keeps its name form the PT 3. The second element keeps its name the PT but ends in -ide

Naming examples Cs 2 O Ba. S Li 3 P

Naming examples Cs 2 O = cesium oxide Ba. S = barium sulfide Li 3 P = lithium phosphide
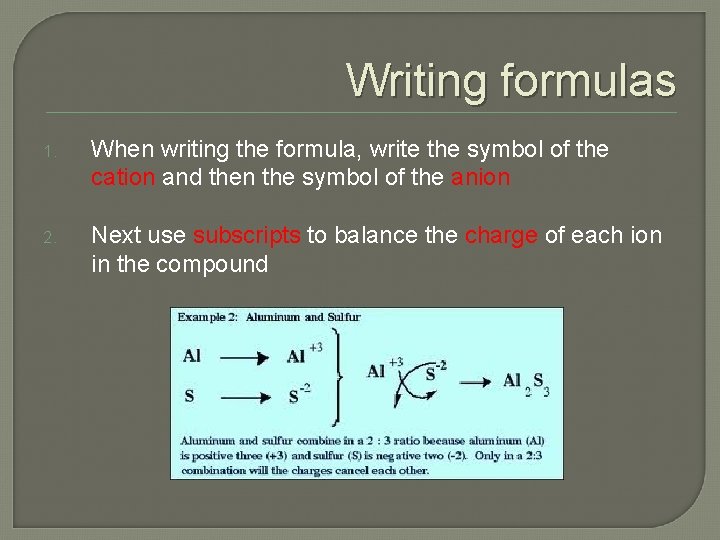
Writing formulas 1. When writing the formula, write the symbol of the cation and then the symbol of the anion 2. Next use subscripts to balance the charge of each ion in the compound

Writing Formulas 1. 2. 3. 4. Write the formulas for compounds formed from these pairs of ions; Ba 2+, S 2 Ca 2+, N 3 Li+, O 2 Al 3+, INow provide the names of each of the cmpds you created

Writing Formulas 1. 2. 3. 4. Write the formulas for compounds formed from these pairs of ions; Ba 2+, S 2 - = Ba. S – barium sulfide Ca 2+, N 3 - = Ca 3 N 2 – calcium nitride Li+, O 2 - = Li 2 O – lithium oxide Al 3+, I- = Al. I 3 – aluminum iodide
- Slides: 20