Lewis Dot Structures Mr Garcia Valence Electrons Valence



- Slides: 18

Lewis Dot Structures Mr. Garcia

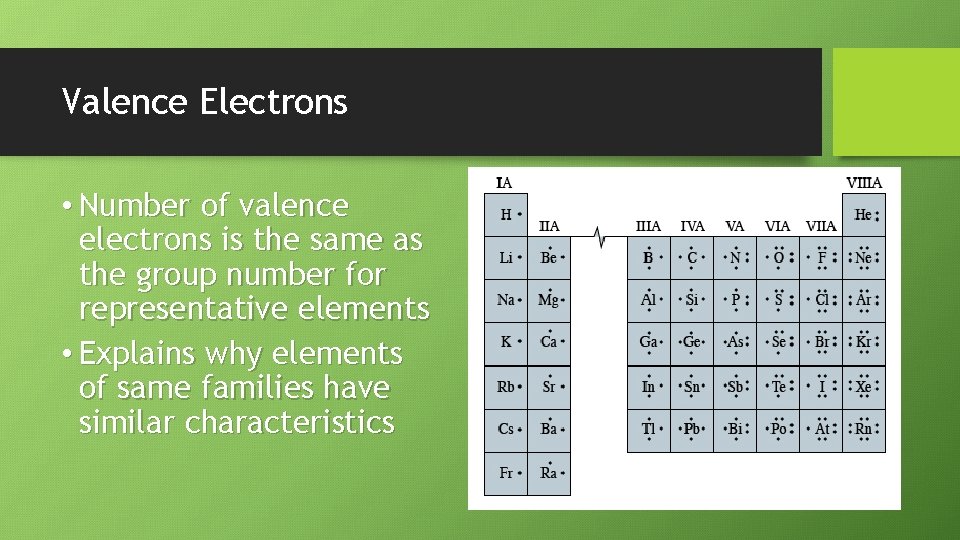
Valence Electrons • Valence electrons – electrons in an atom’s highest energy level • Outermost electrons of the electron cloud • Gives elements chemical characteristics • Only electrons shown in Lewis electron dot structures • Symbolized as dots

Valence Electrons • Number of valence electrons is the same as the group number for representative elements • Explains why elements of same families have similar characteristics

Valence Electrons • Most elements gain or lose valence electrons to have noble gas stability • Noble gas stability- Having full outer shell of electrons • Example: Sodium react violently with chlorine to make Na. Cl (table salt).

Drawing Lewis Dot Structures • Step 1: Identify the symbol for the element and its number of valence electrons using the periodic table. • Step 2: Put the number of electron dots around the symbol. Begin by assigning one dot per side, moving clockwise around the symbol. Start adding a second dot to each side until all of the valence electrons have been placed

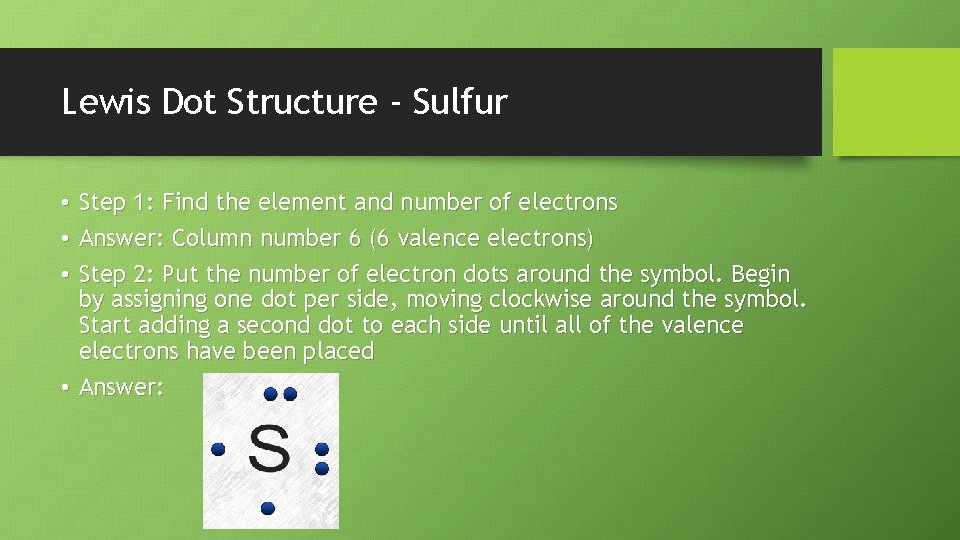
Lewis Dot Structure - Sulfur • Step 1: Find the element and number of electrons • Answer: Column number 6 (6 valence electrons) • Step 2: Put the number of electron dots around the symbol. Begin by assigning one dot per side, moving clockwise around the symbol. Start adding a second dot to each side until all of the valence electrons have been placed • Answer:

Compounds • Line up single dots to form compounds and to make either 2 or 8 electrons around each element. The elements share their single unpaired electrons.

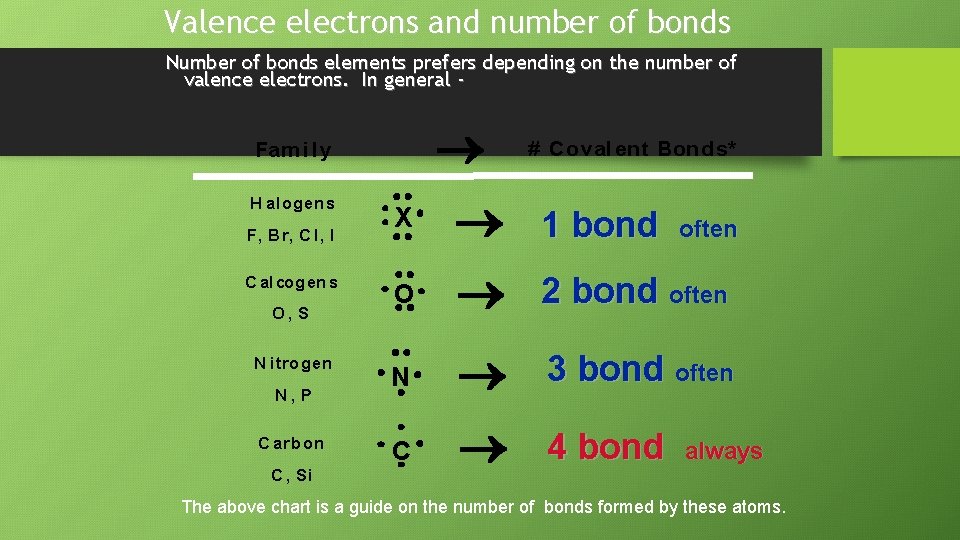
Valence electrons and number of bonds Number of bonds elements prefers depending on the number of valence electrons. In general - Fam i l y H alogen s F, Br, C l, I C alcogen s O, S N itrogen N, P C arb on C , Si # C oval ent Bonds* 1 bond O N 3 bond often C 4 bond X often 2 bond often always The above chart is a guide on the number of bonds formed by these atoms.

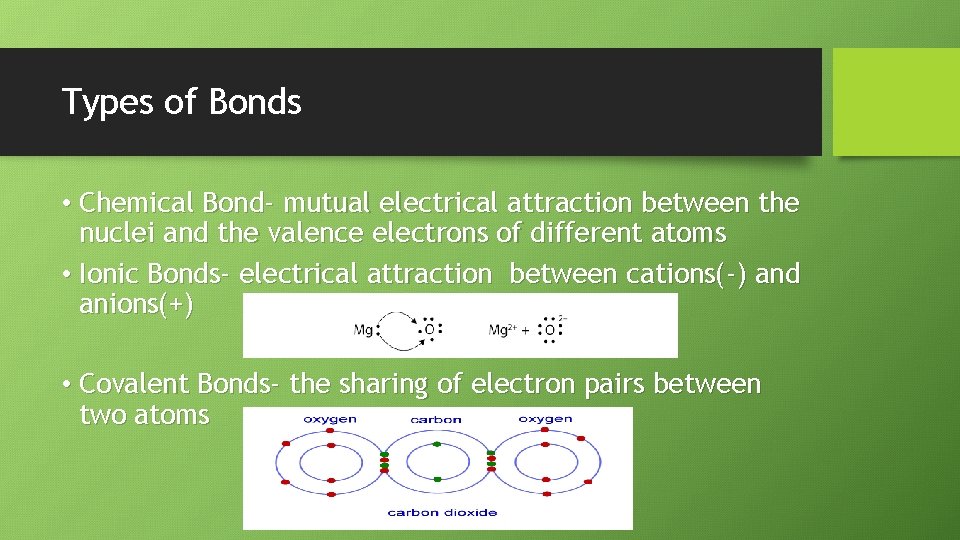
Types of Bonds • Chemical Bond- mutual electrical attraction between the nuclei and the valence electrons of different atoms • Ionic Bonds- electrical attraction between cations(-) and anions(+) • Covalent Bonds- the sharing of electron pairs between two atoms

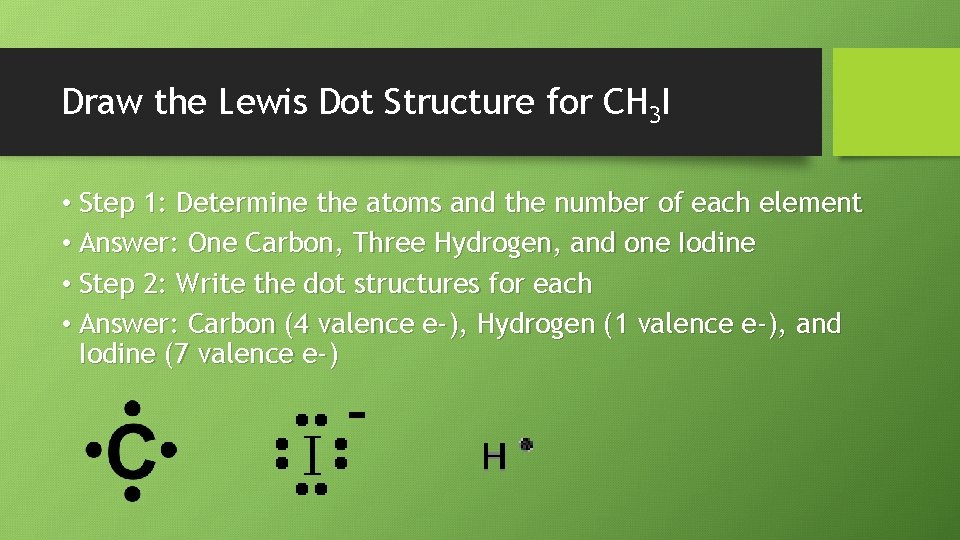
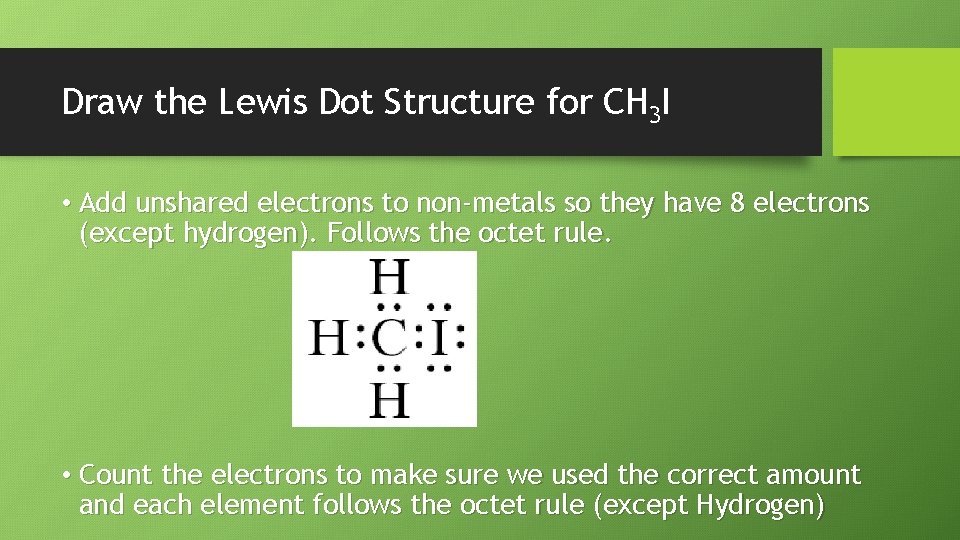
Draw the Lewis Dot Structure for CH 3 I • Step 1: Determine the atoms and the number of each element • Answer: One Carbon, Three Hydrogen, and one Iodine • Step 2: Write the dot structures for each • Answer: Carbon (4 valence e-), Hydrogen (1 valence e-), and Iodine (7 valence e-)

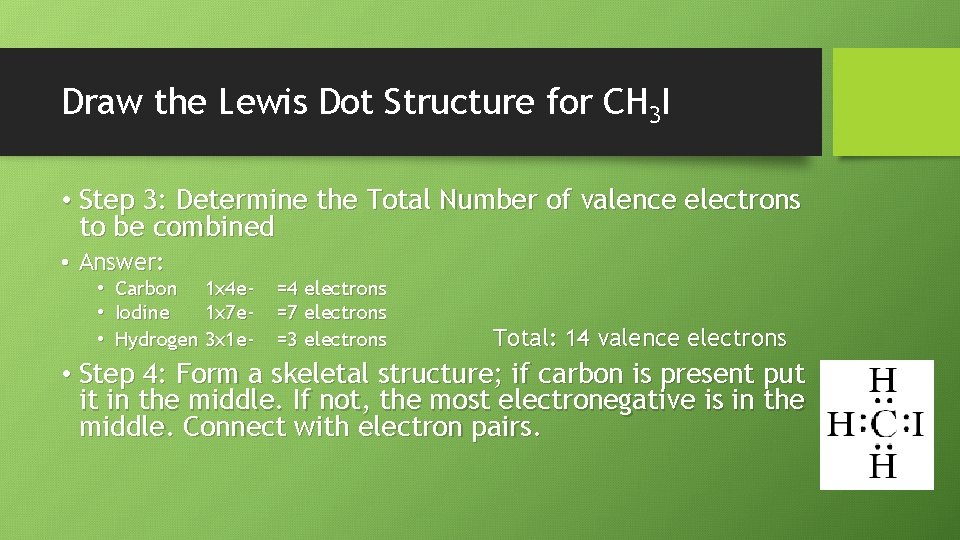
Draw the Lewis Dot Structure for CH 3 I • Step 3: Determine the Total Number of valence electrons to be combined • Answer: • Carbon 1 x 4 e • Iodine 1 x 7 e • Hydrogen 3 x 1 e- =4 electrons =7 electrons =3 electrons Total: 14 valence electrons • Step 4: Form a skeletal structure; if carbon is present put it in the middle. If not, the most electronegative is in the middle. Connect with electron pairs.

Draw the Lewis Dot Structure for CH 3 I • Add unshared electrons to non-metals so they have 8 electrons (except hydrogen). Follows the octet rule. • Count the electrons to make sure we used the correct amount and each element follows the octet rule (except Hydrogen)

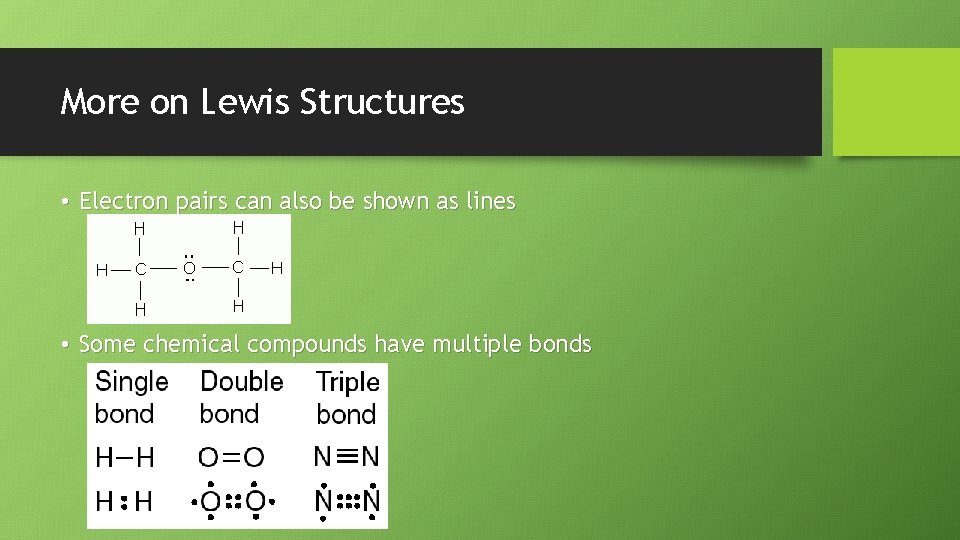
More on Lewis Structures • Electron pairs can also be shown as lines • Some chemical compounds have multiple bonds

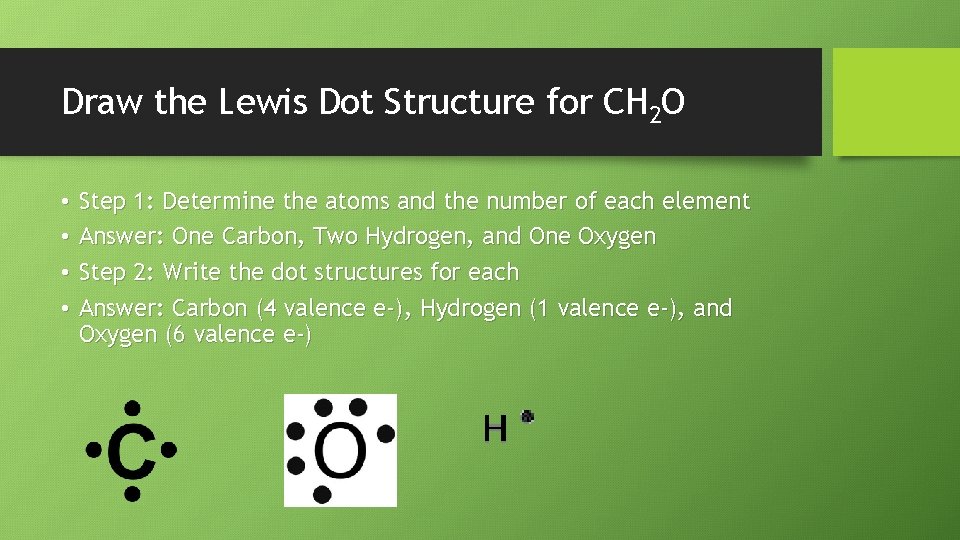
Draw the Lewis Dot Structure for CH 2 O • • Step 1: Determine the atoms and the number of each element Answer: One Carbon, Two Hydrogen, and One Oxygen Step 2: Write the dot structures for each Answer: Carbon (4 valence e-), Hydrogen (1 valence e-), and Oxygen (6 valence e-)

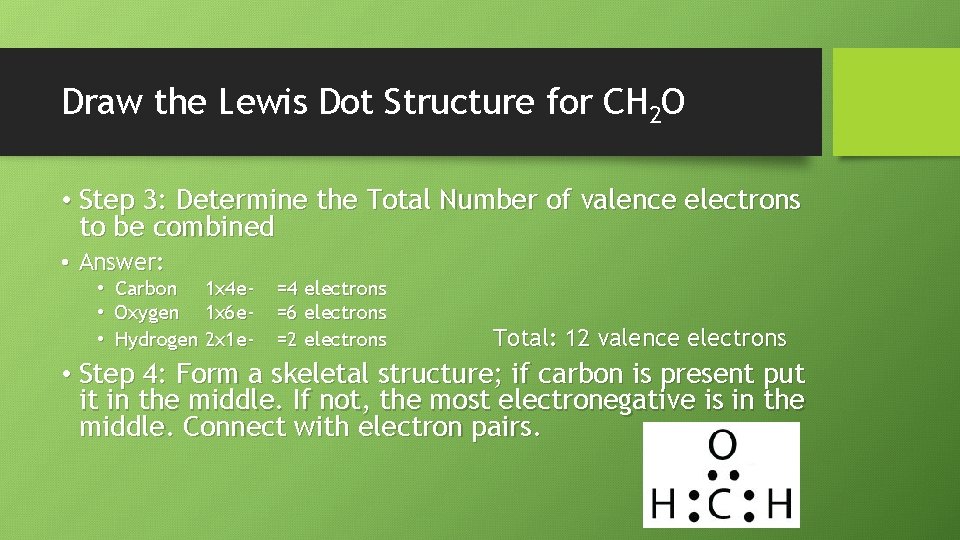
Draw the Lewis Dot Structure for CH 2 O • Step 3: Determine the Total Number of valence electrons to be combined • Answer: • Carbon 1 x 4 e • Oxygen 1 x 6 e • Hydrogen 2 x 1 e- =4 electrons =6 electrons =2 electrons Total: 12 valence electrons • Step 4: Form a skeletal structure; if carbon is present put it in the middle. If not, the most electronegative is in the middle. Connect with electron pairs.

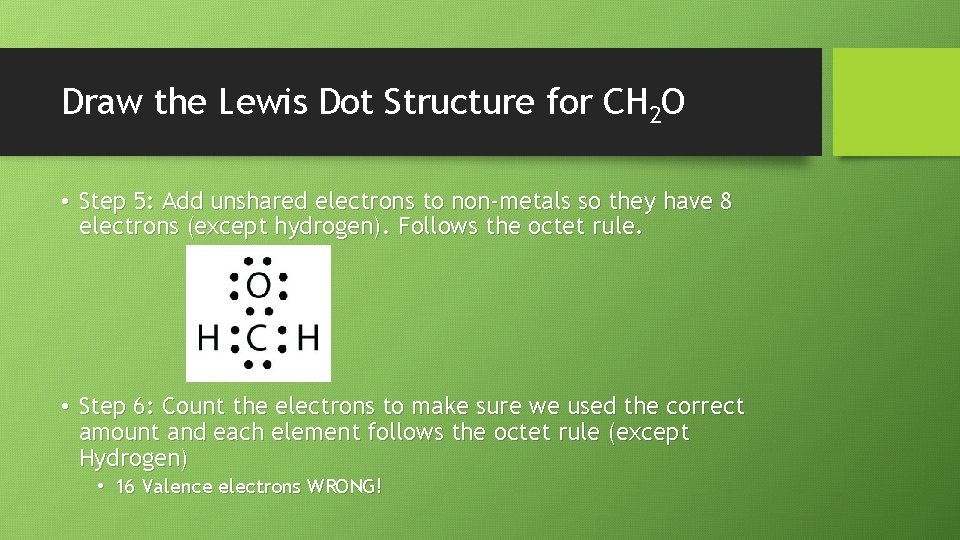
Draw the Lewis Dot Structure for CH 2 O • Step 5: Add unshared electrons to non-metals so they have 8 electrons (except hydrogen). Follows the octet rule. • Step 6: Count the electrons to make sure we used the correct amount and each element follows the octet rule (except Hydrogen) • 16 Valence electrons WRONG!

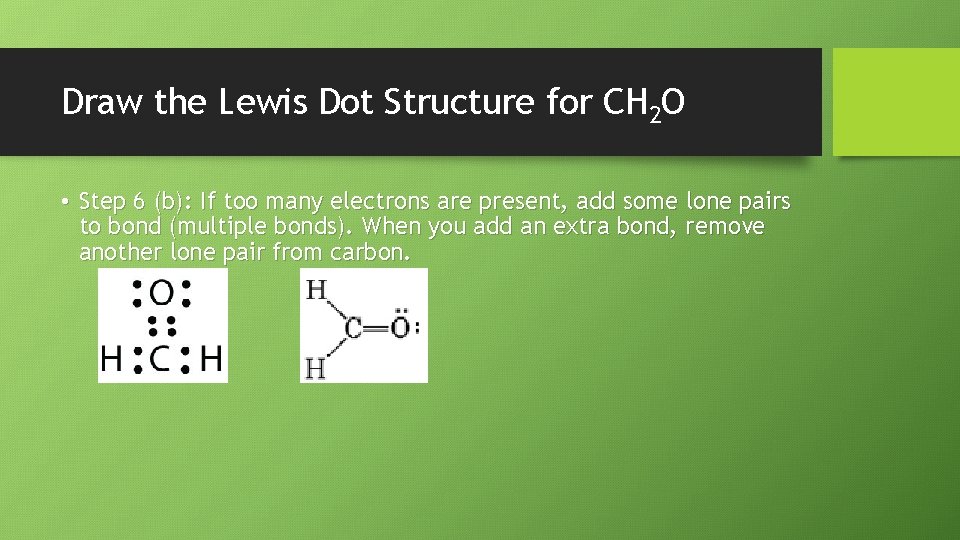
Draw the Lewis Dot Structure for CH 2 O • Step 6 (b): If too many electrons are present, add some lone pairs to bond (multiple bonds). When you add an extra bond, remove another lone pair from carbon.

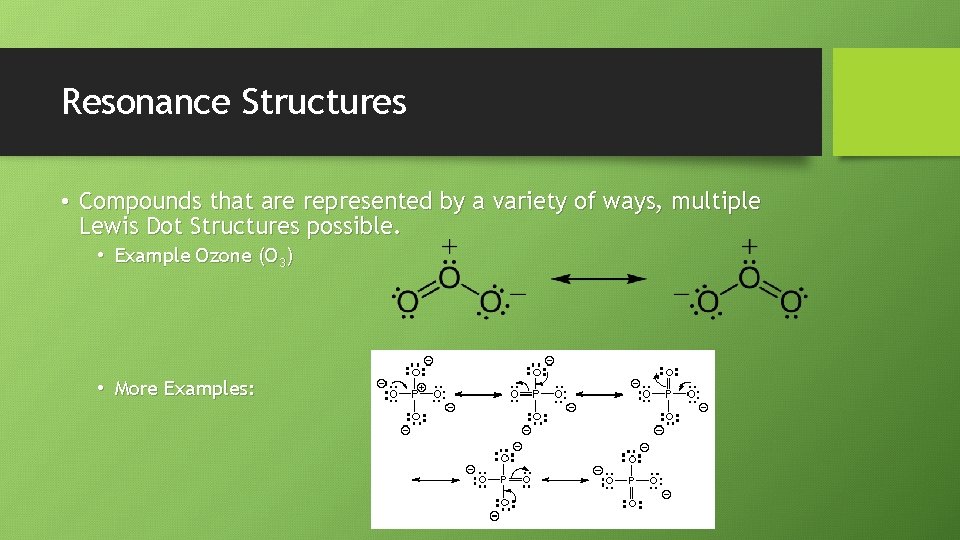
Resonance Structures • Compounds that are represented by a variety of ways, multiple Lewis Dot Structures possible. • Example Ozone (O 3) • More Examples: