Lewis Dot Diagrams and Ions Valence Electrons The

















- Slides: 18

Lewis Dot Diagrams and Ions

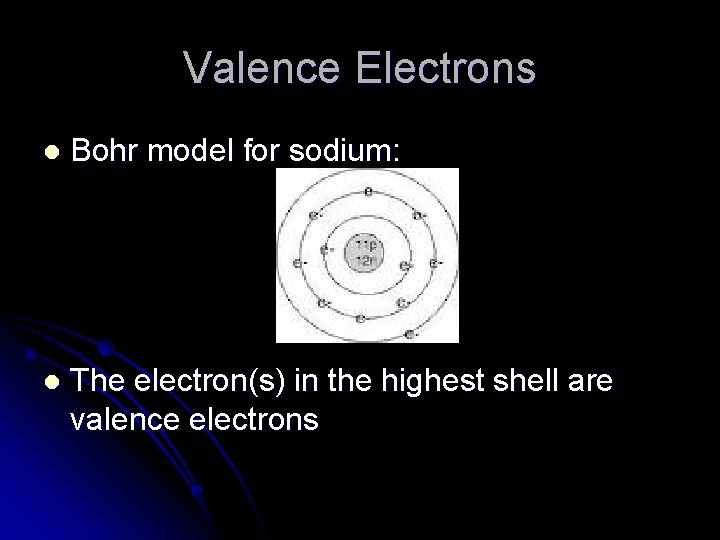
Valence Electrons The valence electrons are the electrons in the outermost shell of the atom l Consider sodium: l How many protons? l How many neutrons? l How many electrons? l Draw a Bohr model for sodium l

Valence Electrons l Bohr model for sodium: l The electron(s) in the highest shell are valence electrons

Lewis Dot Diagram l To draw a Lewis Dot Diagram: l Write the element symbol l Figure out how many valence electrons the atom has l Use dots to represent the valence electrons l Put them in pairs around the 4 sides of the element symbol l For example – try sodium

Lewis Structure Practice l Draw a Lewis Structure for: Calcium Neon Silicon

Ions are atoms that have the same number of protons, but different numbers of electrons l An ion forms when an atom gains or loses electrons to become more stable l Which group is the most stable? l How many valence electrons does that group have? l

Ions When nonmetals form ions, they tend to gain electrons to fill their valence shell with 8 electrons l When metals form ions, they tend to lose all of their valence electrons l

Positive Ion Formation l Atoms in Group 1 lose 1 electron to form ions with a +1 charge l Atoms in Group 2 lose 2 electrons to form ions with a +2 charge l Atoms in Group 13 lose 3 electrons to form ions with a +3 charge l Positive ions are called cations

Negative Ion Formation l Atoms in Group 17 gain 1 electron to form ions with a -1 charge l Atoms in Group 16 gain 2 electrons to form ions with a -2 charge l Atoms in Group 15 gain 3 electrons to form ions with a -3 charge l Negative ions are called anions

Ionic Compounds When atoms form ionic compounds, an atom transfers one or more of its valence electrons to another atom. l Consider Na. Cl l Na loses 1 electron to become Na+1 l Cl gains 1 electron to become Cl-1 l The transfer of electrons from one atom to the other causes a more stable arrangement of electrons. l

Ionic Compounds l May be made from a metal and a nonmetal - KI l May be made from a metal and a polyatomic ion (Look at your reference table) – Li. OH l May be made from two polyatomic ions - NH 4 C 2 H 3 O 2

Naming Ionic Compounds The name of the positive ion stays the same l The end of the element name of the negative ion is changed to an –ide suffix l Chlorine to chloride l Sulfur to sulfide l Phosphorus to phosphide l Polyatomic ions stay as they are written l

Ionic Formulas l What ion does sodium form? l Na+1 l What ion does chlorine form? l Cl-1 l What do we change the name of the negative ion to? l Chloride l What is the name of the compound?

Ionic Formulas What is the formula for the compound made from sodium and chloride ions? l What is the compound made from potassium and iodide ions? l

Ionic Formulas Activity Work in pairs to complete the activity. l Please put all of the shapes back in the bags when you are finished. l

Rules for Naming Ionic Compounds l The first element keeps its name. l For monatomic ions, the second element gets the –ide suffix (ending). l Polyatomic ions always keep their names whether they are first or second.

Practice l Name the following ionic compounds: KF Mg. Br 2 Li 2 S Ca 3 N 2 Na. NO 3 Mg(OH)2

Exit Ticket l Draw a Lewis Structure for magnesium l Give the charge on a magnesium ion l Give the formula for a compound made from magnesium and bromine l Give the name for Ca. Cl 2