Lewis Diagrams Lewis Diagrams Are a way of






- Slides: 12

Lewis Diagrams

Lewis Diagrams � Are a way of representing atoms and their electrons � Only the electrons in the valence shell are shown � Only the valence electrons are shown because they are responsible for the chemical properties of the atom

� Electrons are represented by dots around the symbol of the element � The maximum number of dots is 8 � Hydrogen and Helium have a maximum of 2 dots

Rules for Drawing Lewis Diagrams � Draw the symbol for the element. � The symbol is assumed to have 4 sides and the electrons are distributed around the sides. � The electrons are distributed so that we first place one dot on each side, before we fill in the second dot. � There are no more than 2 electrons per side.

Examples: �Draw the electron dot diagrams for the following: › sodium › beryllium › carbon › fluorine › nitrogen › argon

Lewis Diagrams for Ions � Ions are atoms that have either lost or gained electrons. � They can have either a positive or negative charge. � A positively charged ion has lost at least one electron � A negatively charged ion has gained at least one electron.

Forming Positive Ions � For positive ions, the extra electron or electrons are taken away from the original Lewis Diagram � Positive ions are called cations � When atoms lose electrons, the ion is positive because there are more protons than electrons � Metals typically form positive ions.


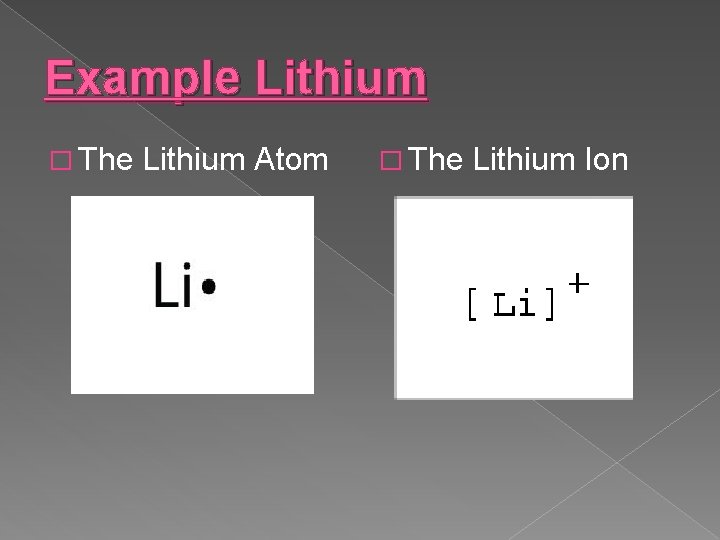
Example Lithium � The Lithium Atom � The Lithium Ion

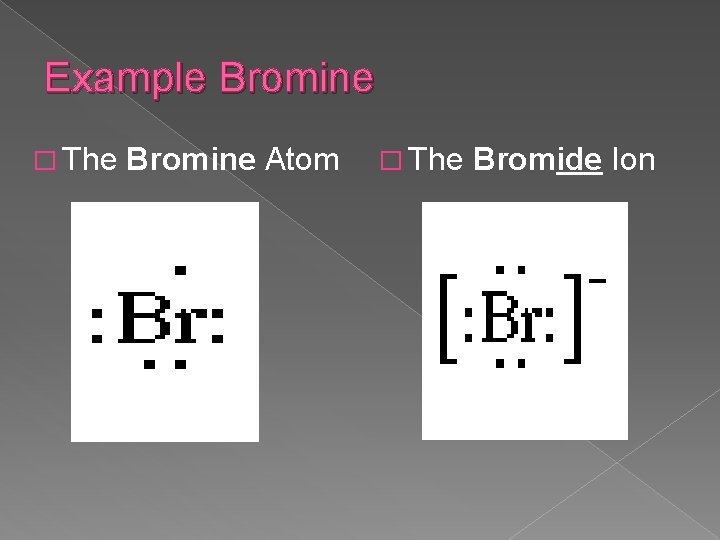
Forming Negative Ions � For negative ions, the extra electron or electrons are added to the original Lewis Diagram � Negative ions are called anions � When atoms gain electrons, the ion is negative because there are more electrons than protons � Non-metals typically form negative ions.

Example Bromine � The Bromine Atom � The Bromide Ion
