Lewis Diagrams Bonding with Lewis Diagrams DEFINITIONS BONDING
Lewis Diagrams
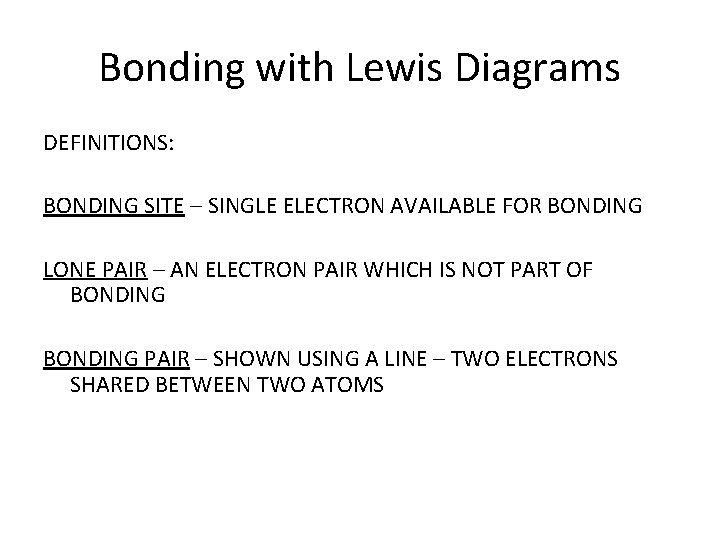
Bonding with Lewis Diagrams DEFINITIONS: BONDING SITE – SINGLE ELECTRON AVAILABLE FOR BONDING LONE PAIR – AN ELECTRON PAIR WHICH IS NOT PART OF BONDING PAIR – SHOWN USING A LINE – TWO ELECTRONS SHARED BETWEEN TWO ATOMS

Bonding with Lewis Diagrams TO SHOW BONDING: • Draw a line between atoms to show they are sharing electrons. • A line represents 2 electrons. • This is also a BONDING PAIR. DOUBLE COUNTING – BOTH ELECTRONS IN THE BONDING PAIR ARE COUNTED AS IN EACH ATOM’S VALENCE SHELL
Examples • Hydrogen and oxygen • Phosphorus and chlorine

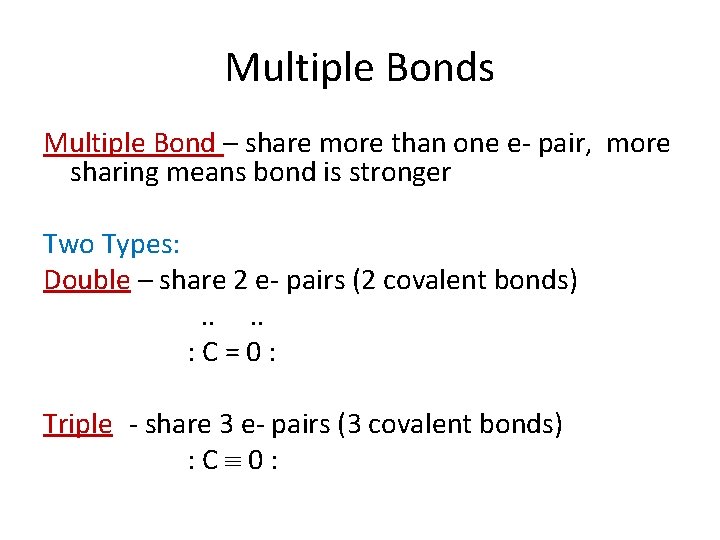
Multiple Bonds Multiple Bond – share more than one e- pair, more sharing means bond is stronger Two Types: Double – share 2 e- pairs (2 covalent bonds). . : C=0: Triple - share 3 e- pairs (3 covalent bonds) : C 0:

Steps Example: HCN 1. Determine the total number of valence electrons (dots) that will be in the final diagram. H – 1 valence e. C – 4 valence e. N – 5 valence e-
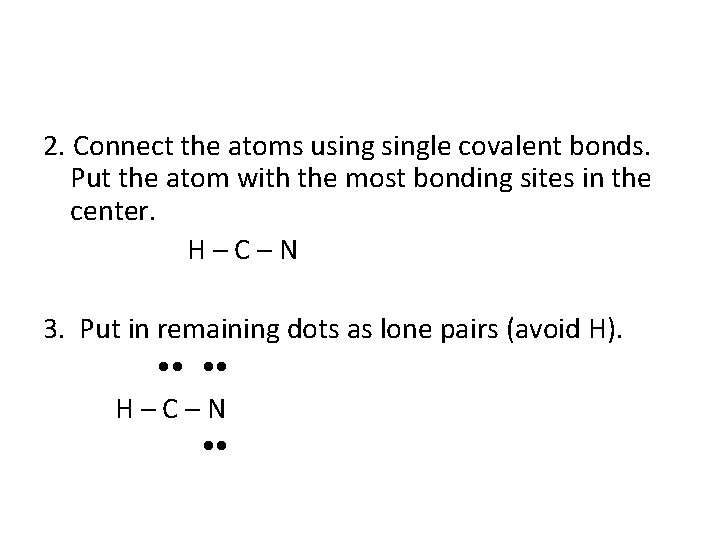
2. Connect the atoms usingle covalent bonds. Put the atom with the most bonding sites in the center. H–C–N 3. Put in remaining dots as lone pairs (avoid H). H–C–N
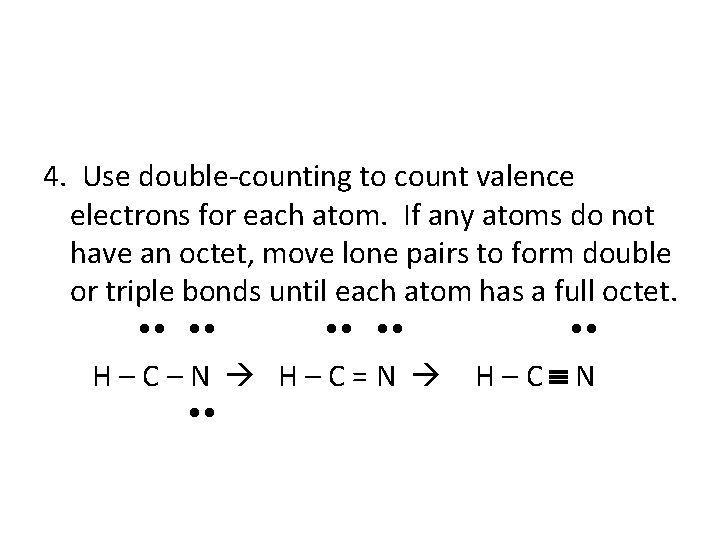
4. Use double-counting to count valence electrons for each atom. If any atoms do not have an octet, move lone pairs to form double or triple bonds until each atom has a full octet. H–C–N H–C=N H–C N

Examples Ge. O 2 O 3
Polyatomic Ions – ions made from covalently bonded atoms • Use parenthesis to show which atoms are included in the polyatomic ion • Ion charge tells you how many electrons to add or take away from Lewis Dot
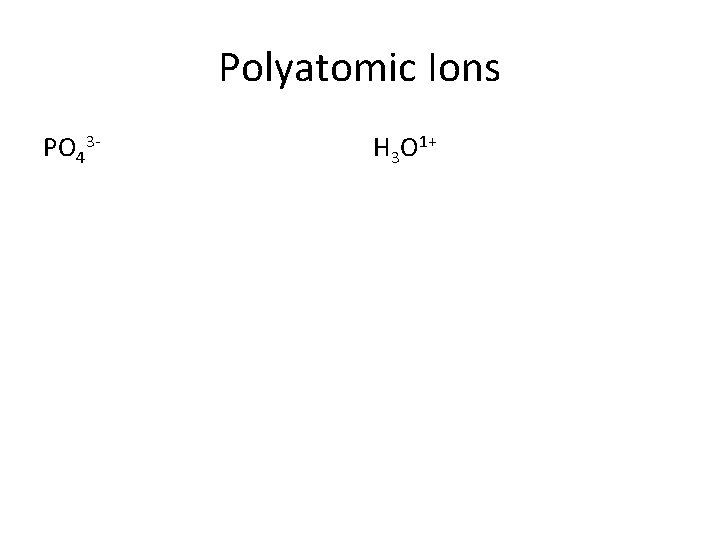
Polyatomic Ions PO 43 - H 3 O 1+
8. 6 Resonance forms – Lewis diagrams which differ only in electron placement (equivalent) Example: O 3
- Slides: 13