Levoluzione della terapia medica dagli analoghi della somatostatina


























- Slides: 53

L’evoluzione della terapia medica: dagli analoghi della somatostatina alle terapie a bersaglio molecolare Salvatore Artale Oncologia Medica Falck Ospedale NIguarda Ca’ Granda Milano 25. 05. 2007

WHO Classification 2000 n Well differentiated endocrine tumors (benign or low grade malignancy) n Well differentiated endocrine carcinomas n Poorly differentiated endocrine carcinomas (small cell carcinomas) n Mixed exocrine and endocrine carcinomas n Tumor-like lesions

WHO Classification 2000 THE DIFFERENTIATION IS BASED ON : HISTOMORPHOLOGY PRESENCE/ABSENCE OF LOC. INVASION/METASTASIS PROLIFERATION INDEX (Ki 67): < 2% Well Diff. Tumors > 2% / <15% Well. Diff. Carcinomas > 15 % Poorly Diff. Carcinomas

Neuroendocrine tumors Frequency Incidence: 1 -2/ 100. 000 Autopsy series 8/100. 000 Carcinoid tumors are the most frequent type (40% of all NETs)

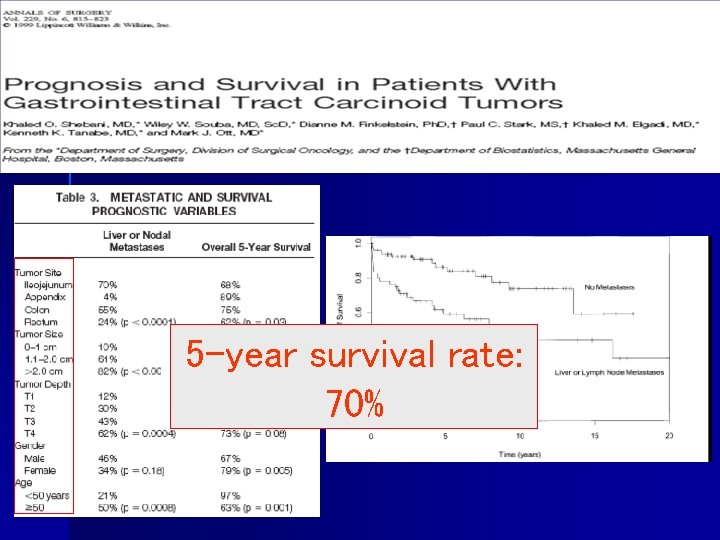
5 -year survival rate: 70%

WHO Classification 2000 n Well differentiated endocrine tumors (benign or low grade malignancy) n Well differentiated endocrine carcinomas n Poorly differentiated endocrine carcinomas (small cell carcinomas) n Mixed exocrine and endocrine carcinomas n Tumor-like lesions

WHO Classification 2000 n Well differentiated endocrine carcinomas Which is the best treatment ?

Medical treatment n Biotherapy ? n Chemotherapy ?

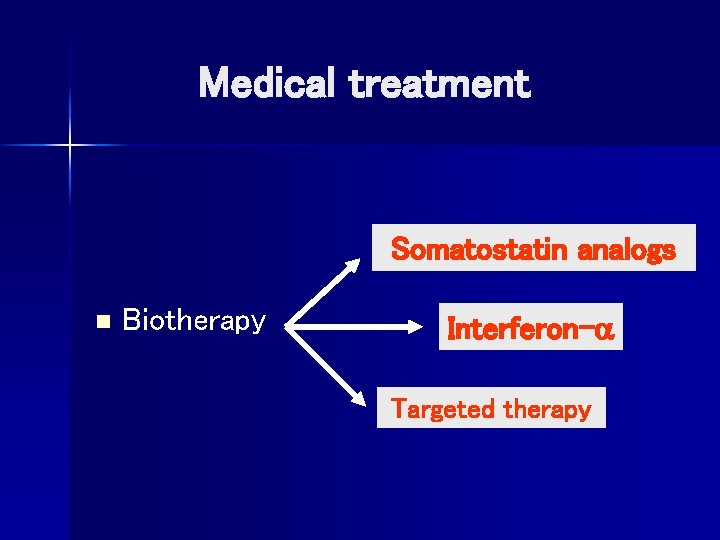
Medical treatment Somatostatin analogs n Biotherapy Interferon- Targeted therapy

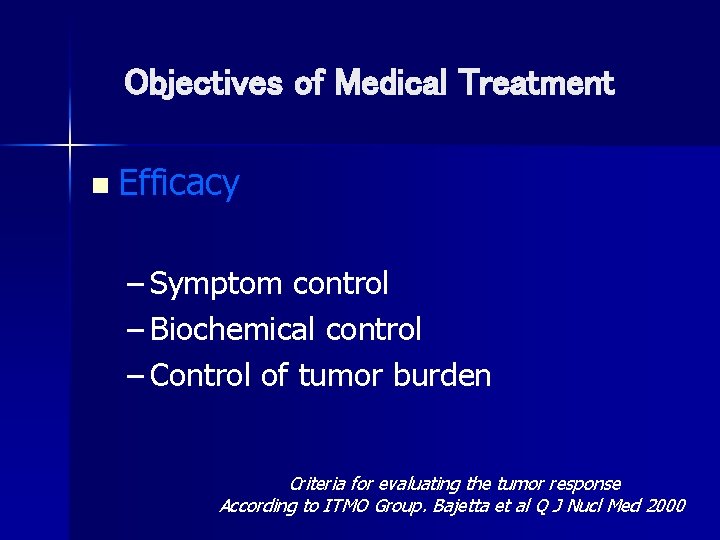
Objectives of Medical Treatment n Efficacy – Symptom control – Biochemical control – Control of tumor burden Criteria for evaluating the tumor response According to ITMO Group. Bajetta et al Q J Nucl Med 2000


Clinical Presentation: non functioning tumours – Symptoms related to the mass effect: – Bowel obstruction – GI bleeding (rare)

Clinical Presentation: functioning tumours – Carcinoid syndrome < 20%: – Cutaneous flushing: upper part of the body (80%) n Watery diarrhea and abdominal cramp (80%) n Bronchospasm n Endocardial fibrosis( 30 -40 %): arrhythmia. Right heart insufficiency.

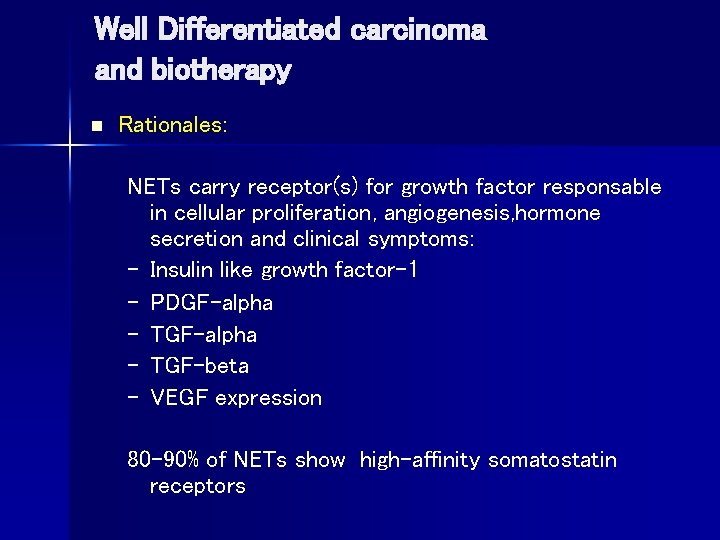
Well Differentiated carcinoma and biotherapy n Rationales: NETs carry receptor(s) for growth factor responsable in cellular proliferation, angiogenesis, hormone secretion and clinical symptoms: – Insulin like growth factor-1 – PDGF-alpha – TGF-beta – VEGF expression 80 -90% of NETs show high-affinity somatostatin receptors

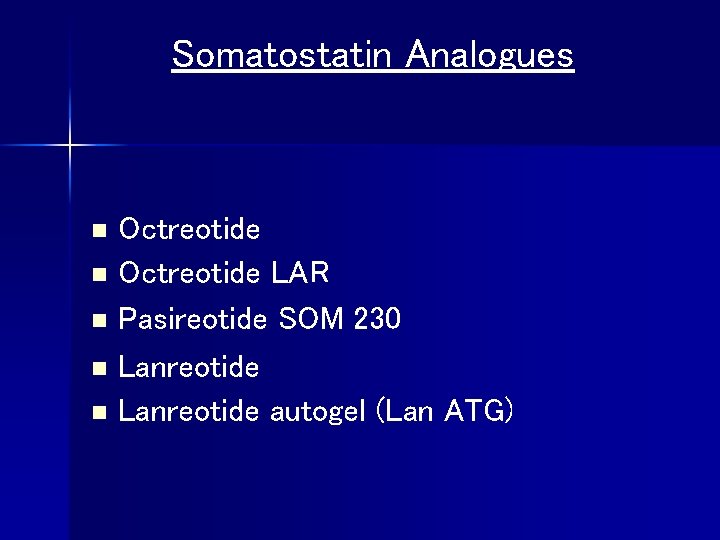
Somatostatin Analogues Octreotide n Octreotide LAR n Pasireotide SOM 230 n Lanreotide autogel (Lan ATG) n

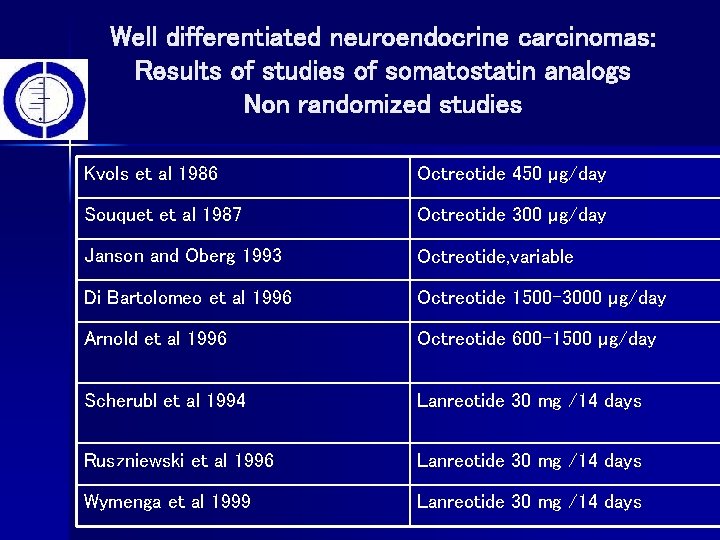
Well differentiated neuroendocrine carcinomas: Results of studies of somatostatin analogs Non randomized studies Kvols et al 1986 Octreotide 450 µg/day Souquet et al 1987 Octreotide 300 µg/day Janson and Oberg 1993 Octreotide, variable Di Bartolomeo et al 1996 Octreotide 1500 -3000 µg/day Arnold et al 1996 Octreotide 600 -1500 µg/day Scherubl et al 1994 Lanreotide 30 mg /14 days Ruszniewski et al 1996 Lanreotide 30 mg /14 days Wymenga et al 1999 Lanreotide 30 mg /14 days

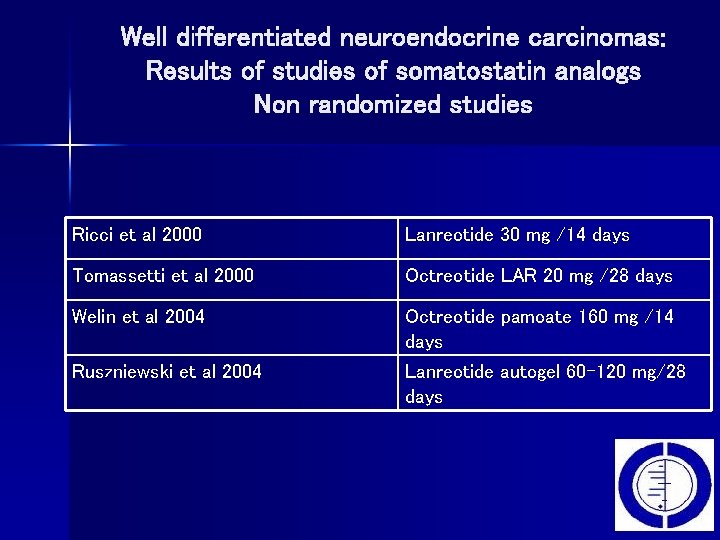
Well differentiated neuroendocrine carcinomas: Results of studies of somatostatin analogs Non randomized studies Ricci et al 2000 Lanreotide 30 mg /14 days Tomassetti et al 2000 Octreotide LAR 20 mg /28 days Welin et al 2004 Octreotide pamoate 160 mg /14 days Lanreotide autogel 60 -120 mg/28 days Ruszniewski et al 2004

Somatostatin analogs SR: 30 -75% OR: 5% Standard dose BR: 30 -60% SD: 35 -50% Med Dur SD: 18 months

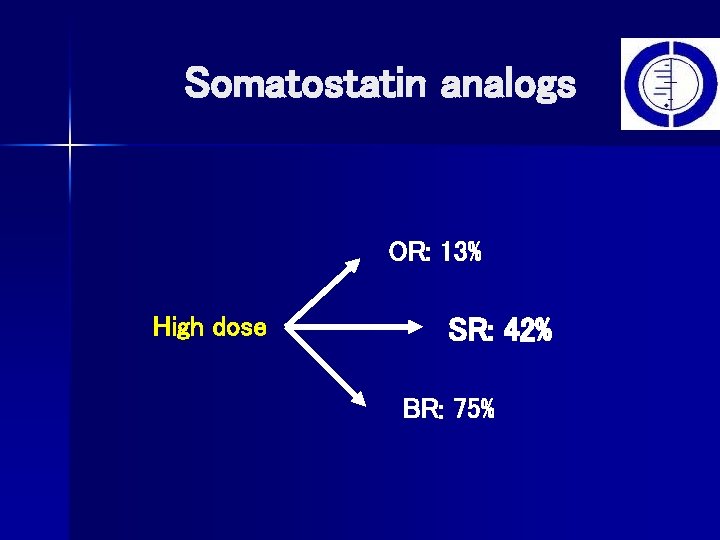
Somatostatin analogs OR: 13% High dose SR: 42% BR: 75%

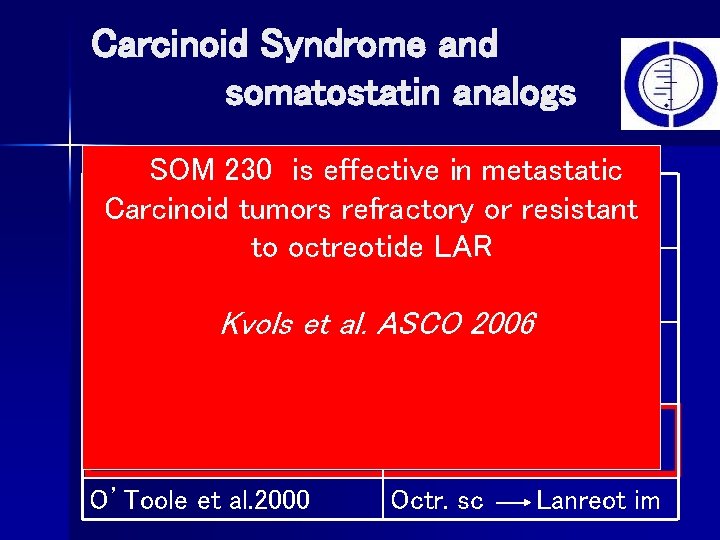
Carcinoid Syndrome and somatostatin analogs Efficacy Studies Randomized trials: 5

Carcinoid Syndrome and somatostatin analogs Randomized trials Lanreotide ATG SOM 230 is effective 120 in mg metastatic Oberg et al. 61989 Octreotide scmg vs every weeks = Lanreotide Carcinoid tumors refractory or 60 resistant placebo every 3 weeks to octreotide LAR in well neuroendocrine Jacobsen anddifferentiated Hanssen Octreotide sc vs tumors 1995 placebo Kvols et al. ASCO 2006 Saslow et al. 1997 Octreotide sc vs Bajetta et al. Cancer 2006 Placebo Rubin et al. 1999 Octreotide LAR vs Octreotide sc O’Toole et al. 2000 Octr. sc Lanreot im

What about interferon ?

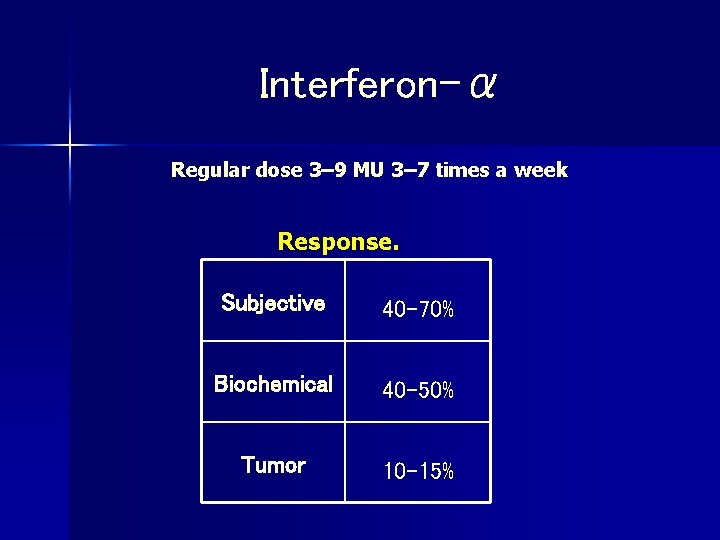
Interferon-α Regular dose 3– 9 MU 3– 7 times a week Response. Subjective 40– 70% Biochemical 40– 50% Tumor 10– 15%

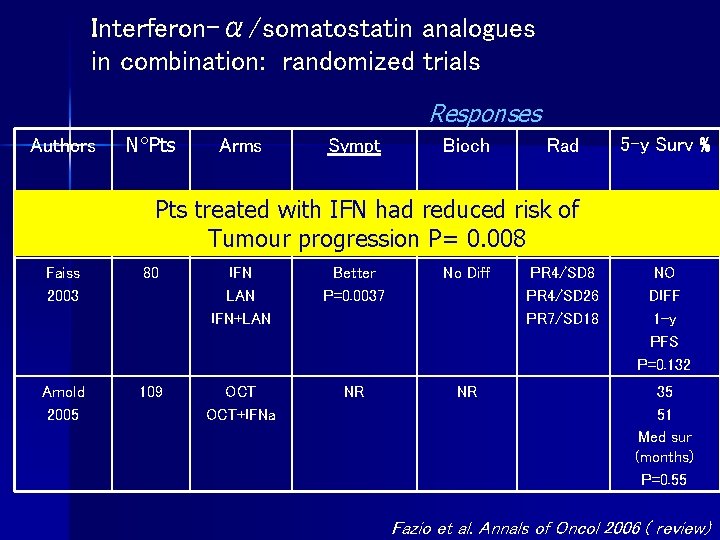
Interferon-α/somatostatin analogues in combination: randomized trials Responses Authors N°Pts Arms Sympt Bioch Kolby 2003 68 Pts Faiss 2003 80 IFN LAN IFN+LAN Better P=0. 0037 No Diff Arnold 2005 109 OCT+IFNa NR NR Rad IFN NR had reduced NR treated with IFN risk NR of OCT+IFNa Tumour progression P= 0. 008 PR 4/SD 26 PR 7/SD 18 5 -y Surv % 36 58 NO DIFF 1 -y PFS P=0. 132 35 51 Med sur (months) P=0. 55 Fazio et al. Annals of Oncol 2006 ( review)

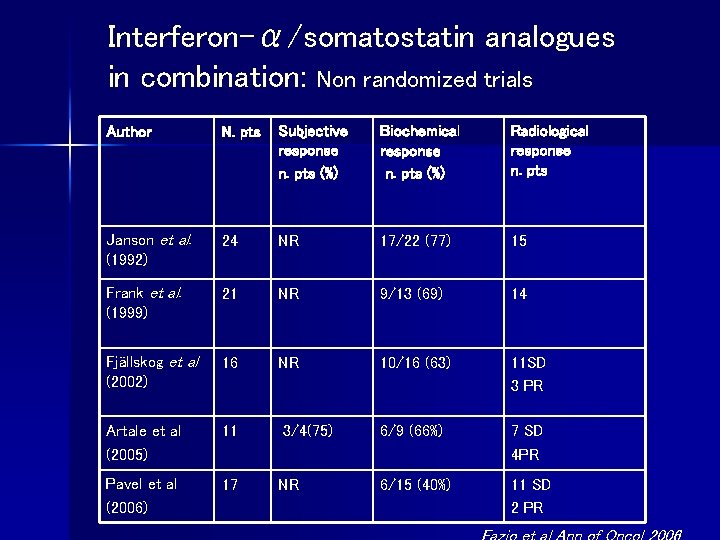
Interferon-α/somatostatin analogues in combination: Non randomized trials Author N. pts Subjective response n. pts (%) Biochemical response n. pts (%) Radiological response n. pts Janson et al. (1992) 24 NR 17/22 (77) 15 Frank et al. (1999) 21 NR 9/13 (69) 14 Fjällskog et al (2002) 16 NR 10/16 (63) 11 SD 3 PR Artale et al (2005) 11 6/9 (66%) 7 SD 4 PR Pavel et al (2006) 17 6/15 (40%) 11 SD 2 PR 3/4(75) NR

WHO Classification 2000 n Well differentiated endocrine tumors (benign or low grade malignancy) n Well differentiated endocrine carcinomas n Poorly differentiated endocrine carcinomas (small cell carcinomas) n Mixed exocrine and endocrine carcinomas n Tumor-like lesions

WHO Classification 2000 n Poorly differentiated endocrine carcinomas Which is the best treatment ?

Medical treatment n Chemotherapy

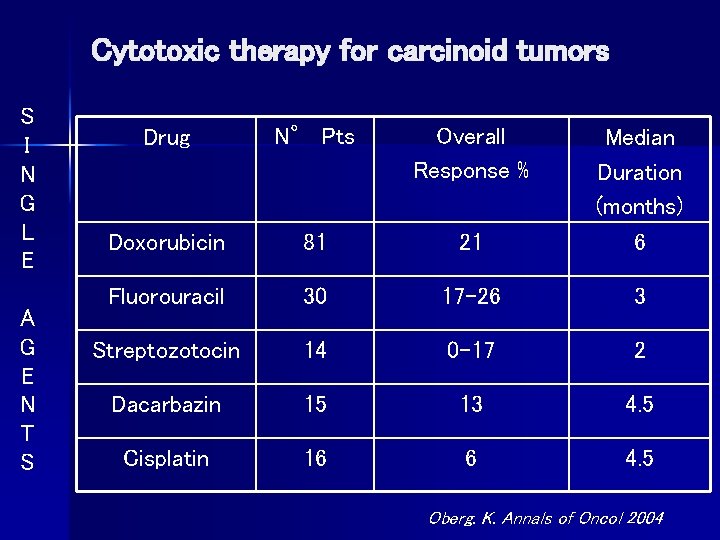
Cytotoxic therapy for carcinoid tumors S I N G L E A G E N T S Drug N° Pts Overall Response % Doxorubicin 81 21 Median Duration (months) 6 Fluorouracil 30 17 -26 3 Streptozotocin 14 0 -17 2 Dacarbazin 15 13 4. 5 Cisplatin 16 6 4. 5 Oberg. K. Annals of Oncol 2004

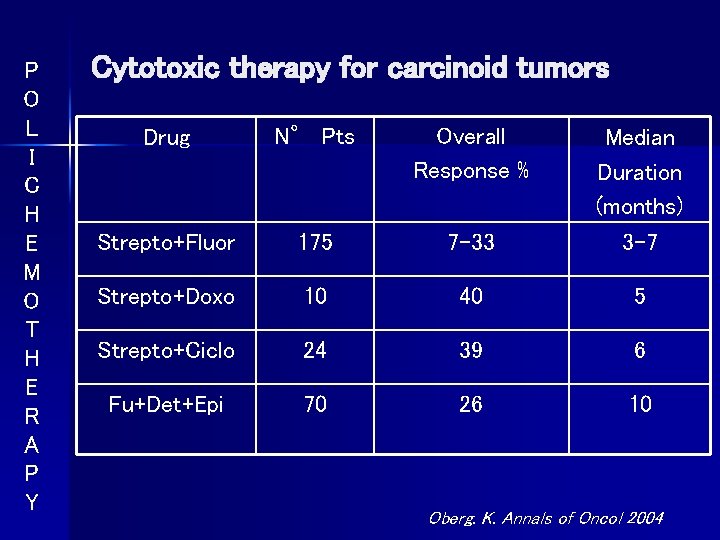
P O L I C H E M O T H E R A P Y Cytotoxic therapy for carcinoid tumors Drug N° Pts Overall Response % Strepto+Fluor 175 7 -33 Median Duration (months) 3 -7 Strepto+Doxo 10 40 5 Strepto+Ciclo 24 39 6 Fu+Det+Epi 70 26 10 Oberg. K. Annals of Oncol 2004

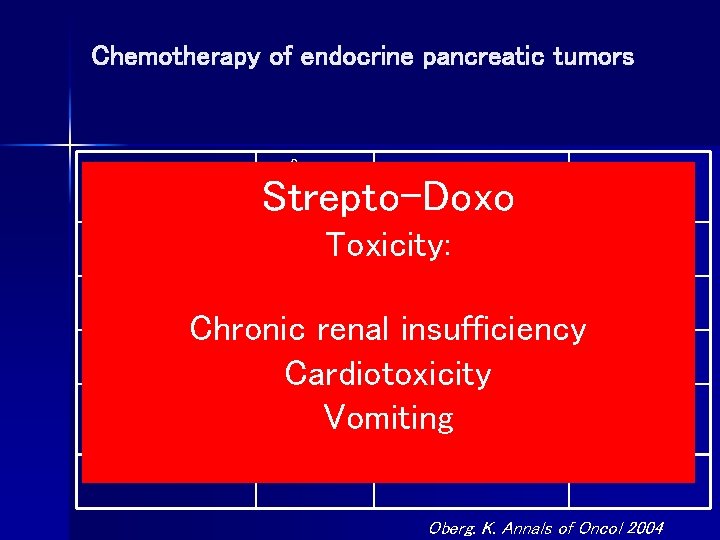
Chemotherapy of endocrine pancreatic tumors Drug N° Pts Objective Response % Strepto-Doxo Strepto+Fluor 170 Toxicity: 45 -63 Strepto+Doxo 50 40 -69 Chronic renal insufficiency Cispl+Etop 14 50 Cardiotoxicity Dacarbazine 11 9 Vomiting Paclitaxel 15 7 Duration (months) 18 -36 12 -24 9 6 5 Oberg. K. Annals of Oncol 2004

Failure to Confirm Major Objective Antitumor Activity for Streptozotocin And Doxorubicin in the treatment of Patients with Advanced Islet Cell Carcinoma MSKCC. 2/92 -2/98 16 patients with ICC treated with STZ + Doxo Results: 1/16 ( 6%) with imaging PR 9/16 (56%) with stable disease 6/16 (38%) progressed during treament Cheng et al Cancer 1999

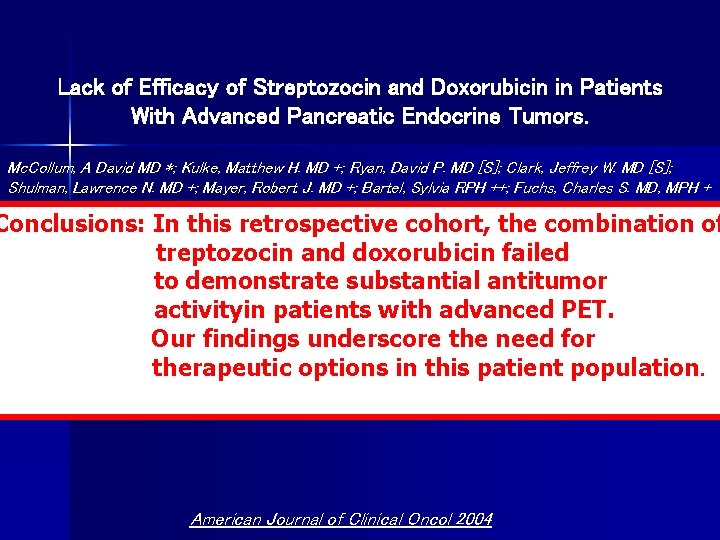
Lack of Efficacy of Streptozocin and Doxorubicin in Patients With Advanced Pancreatic Endocrine Tumors. Mc. Collum, A David MD *; Kulke, Matthew H. MD +; Ryan, David P. MD [S]; Clark, Jeffrey W. MD [S]; Shulman, Lawrence N. MD +; Mayer, Robert J. MD +; Bartel, Sylvia RPH ++; Fuchs, Charles S. MD, MPH + Methods: We retrospectively reviewed the records of 16 consecutive patients who received streptozocin Conclusions: In this retrospective cohort, the combination of and doxorubicin for advanced PETs at Dana Farber/Partners Cancer Care institutions. Baseline patient treptozocin and doxorubicin failed characteristics, radiographic response to therapy, treatment-related toxicity, progression-free and overall survival were analyzed. to demonstrate substantial antitumor activityin patients with advanced PET. Results: One patient demonstrated an objective partial response to therapy (objective response rate [ORR], 6%; 95% confidence interval [CI], 0 -18%). Six patients achieved stable disease (38%; 95% CI, 14 Our findings underscore the need for 62%) and 9 patients demonstrated disease progression on initial restaging (56%; 95% CI, 33 -77%). The therapeutic options in this patient population. median progression-free survival and overall survival were 3. 9 months (95% CI, 2. 8 -8. 8) and 20. 2 months (95% CI, 9. 7 -37. 4), respectively. American Journal of Clinical Oncol 2004

Cisplatin Based Therapy

RESPONSE TO CISPLATIN AND ETOPOSIDE COMBINATION ACCORDING TO CELLULAR DIFFERENTION Well differentiated Poorly differentiated Patient no. 4 11 Overall response % 45 % 67 Biochemical % 45 %0 Radiological % 27 % 50 Stable % 36 % 25 Progressive % 18 %0 Fjallskog Cancer 2001

Well diff Poorly diff

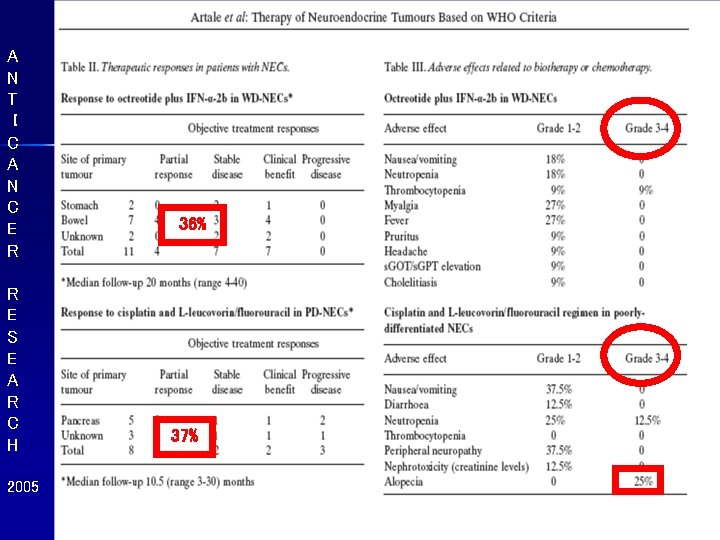
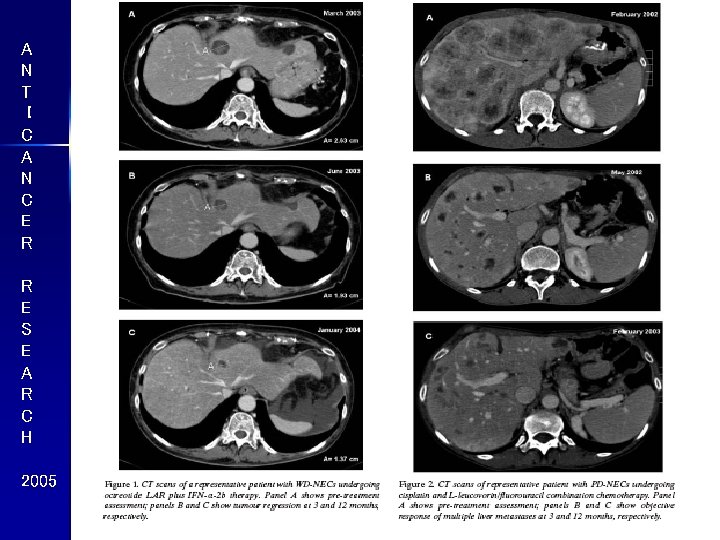
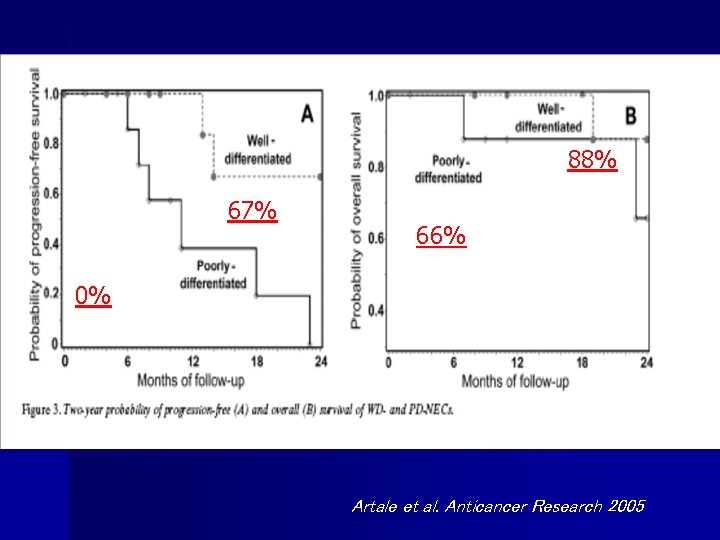
A N T I C A N C E R R E S E A R C H 2005 36% 37%

A N T I C A N C E R R E S E A R C H 2005

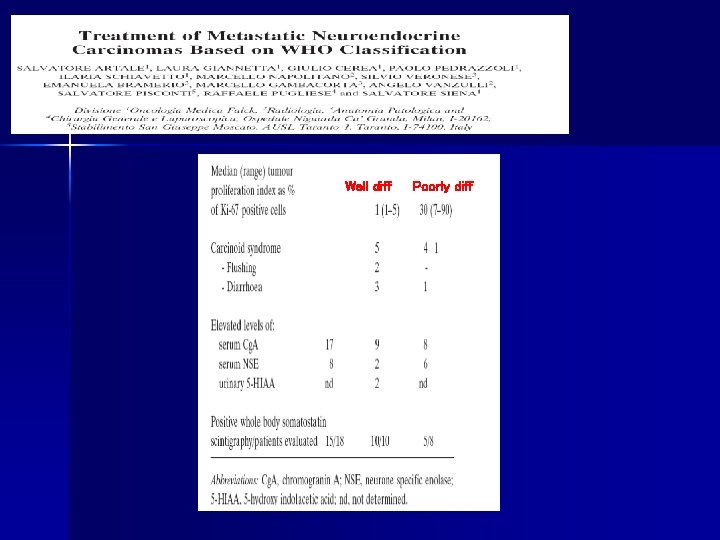
88% 67% 66% 0% Artale et al. Anticancer Research 2005

Targeted Therapy n Monoclonal antibody anti-VEGF: Bevacizumab: suppression of tumor blood flow and prolungation of PFS. Yao et al ASCO 2005 n Small multi-TK inhibitors: Sunitinib: RR 15% islet cell, 2% carcinoid, SD JCO 2006 Sorafenib, Vatalanib : Phase II ongoing Imatinib (Gleevec): no activity Gross et al. Endocrine related Cancer 2006 Endostatin: only SD. Kulke et al JCO 2006 n EGFR inhibitors: Gefitinib: no Object. resp. ASCO 2005

Mammallian target of rapamycin inhibitors EVEROLIMUS (RAD 001) TEMSIROLIMUS ( CCI-779)

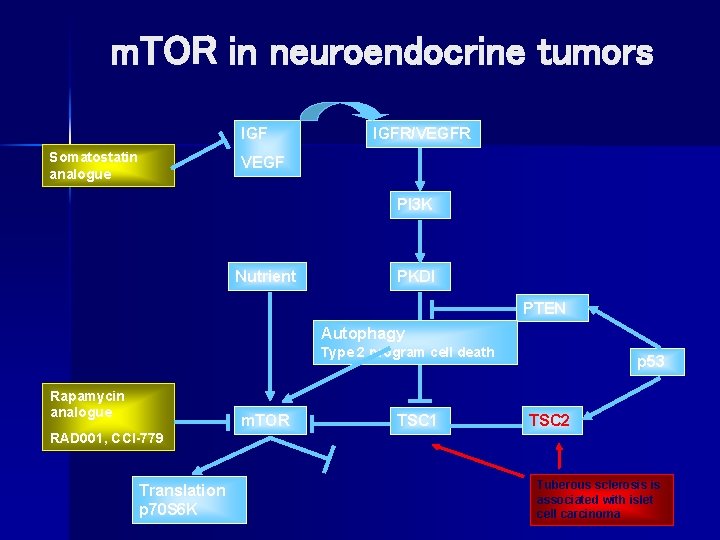
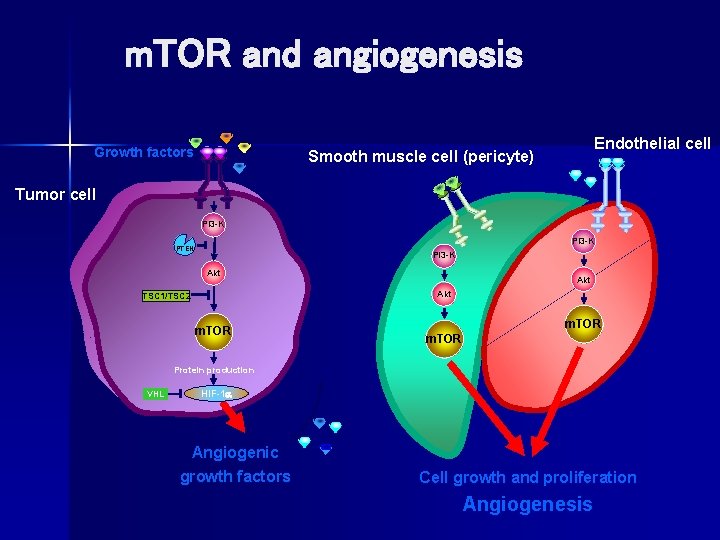
Role of angiogenesis in NET n Angiogenic growth factors contribute to tumor growth – VEGF is found in 84% of carcinoid and 59% of islet cell tumors – VEGFR is found in 71% of carcinoid and 67% of islet cell tumors – Suggests autocrine stimulation in carcinoid and islet cell tumors n The m. TOR pathway regulates production of angiogenic growth factors and the proliferation of vascular endothelial cells Hobday et al. Proc ASCO. 2003; 22: 269. Abstract 1078.

m. TOR in neuroendocrine tumors IGF Somatostatin analogue IGFR/VEGFR VEGF PI 3 K Nutrient PKDI PTEN Autophagy AKTcell death Type 2 program Rapamycin analogue m. TOR TSC 1 p 53 TSC 2 RAD 001, CCI-779 Translation p 70 S 6 K Tuberous sclerosis is associated with islet cell carcinoma

m. TOR and angiogenesis Growth factors Endothelial cell Smooth muscle cell (pericyte) Tumor cell PI 3 -K PTEN PI 3 -K Akt Akt TSC 1/TSC 2 m. TOR Protein production VHL HIF-1 Angiogenic growth factors Cell growth and proliferation Angiogenesis

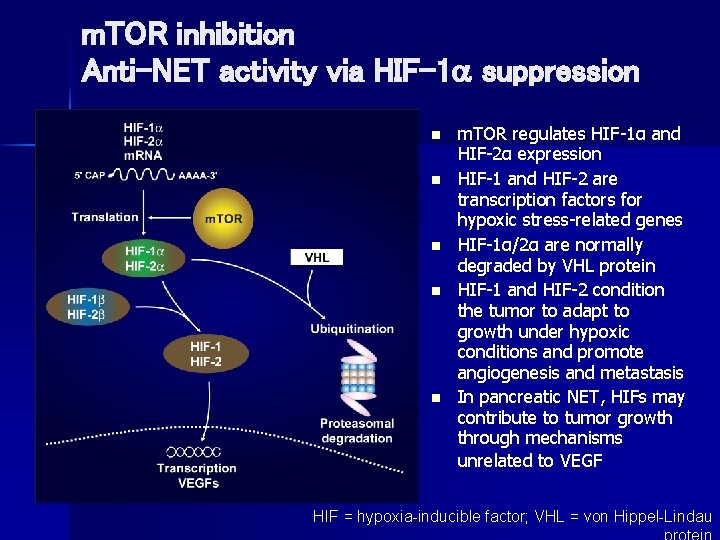
m. TOR inhibition Anti-NET activity via HIF-1 suppression n n m. TOR regulates HIF-1α and HIF-2α expression HIF-1 and HIF-2 are transcription factors for hypoxic stress-related genes HIF-1α/2α are normally degraded by VHL protein HIF-1 and HIF-2 condition the tumor to adapt to growth under hypoxic conditions and promote angiogenesis and metastasis In pancreatic NET, HIFs may contribute to tumor growth through mechanisms unrelated to VEGF HIF = hypoxia-inducible factor; VHL = von Hippel-Lindau

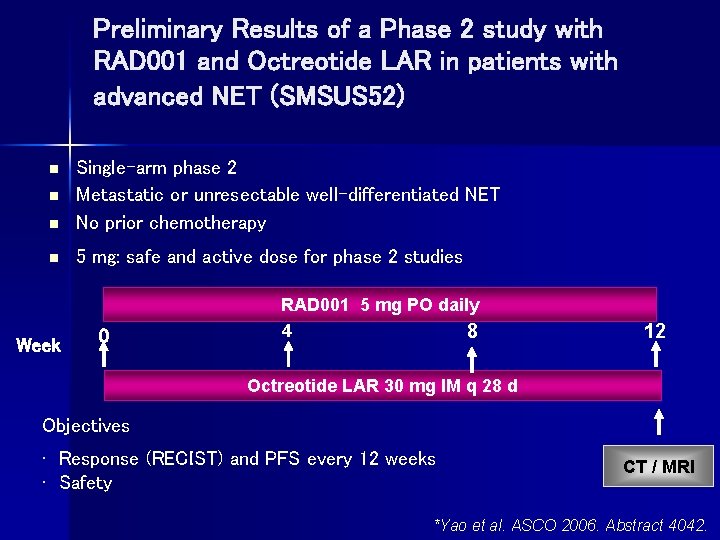
Preliminary Results of a Phase 2 study with RAD 001 and Octreotide LAR in patients with advanced NET (SMSUS 52) n Single-arm phase 2 Metastatic or unresectable well-differentiated NET No prior chemotherapy n 5 mg: safe and active dose for phase 2 studies n n Week 0 RAD 001 5 mg PO daily 4 8 12 Octreotide LAR 30 mg IM q 28 d Objectives • Response (RECIST) and PFS every 12 weeks • Safety CT / MRI *Yao et al. ASCO 2006. Abstract 4042.

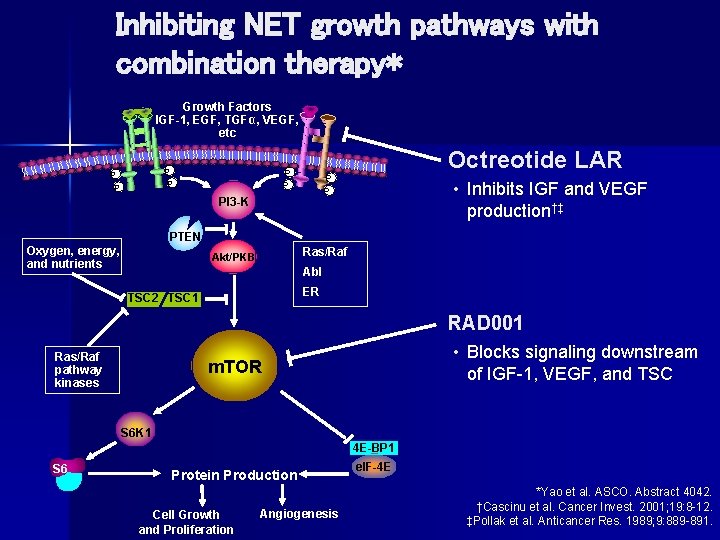
Inhibiting NET growth pathways with combination therapy* Growth Factors IGF-1, EGF, TGFα, VEGF, etc P P P Octreotide LAR P P PI 3 -K • Inhibits IGF and VEGF production†‡ PTEN Oxygen, energy, and nutrients Ras/Raf Akt/PKB Abl ER TSC 2 TSC 1 RAD 001 Ras/Raf pathway kinases • Blocks signaling downstream of IGF-1, VEGF, and TSC m. TOR S 6 K 1 4 E-BP 1 S 6 Protein Production Cell Growth and Proliferation Angiogenesis el. F-4 E *Yao et al. ASCO. Abstract 4042. †Cascinu et al. Cancer Invest. 2001; 19: 8 -12. ‡Pollak et al. Anticancer Res. 1989; 9: 889 -891.

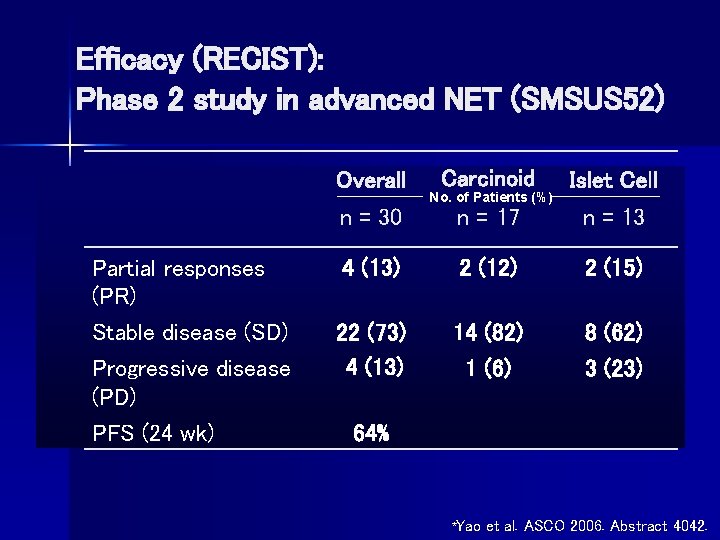
Efficacy (RECIST): Phase 2 study in advanced NET (SMSUS 52) Carcinoid Islet Cell n = 17 n = 13 4 (13) 2 (12) 2 (15) 22 (73) 4 (13) 14 (82) 1 (6) 8 (62) 3 (23) Overall n = 30 Partial responses (PR) Stable disease (SD) Progressive disease (PD) PFS (24 wk) No. of Patients (%) 64% *Yao et al. ASCO 2006. Abstract 4042.

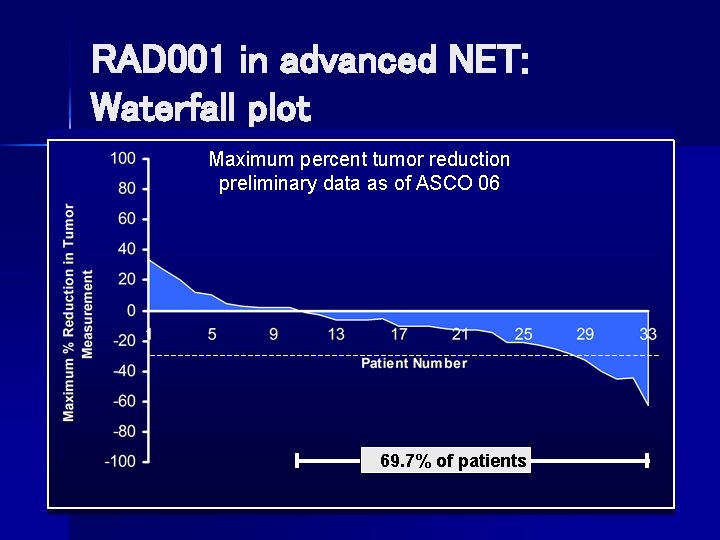
RAD 001 in advanced NET: Waterfall plot Maximum percent tumor reduction preliminary data as of ASCO 06 69. 7% of patients

RAD 001 Safety: Phase 2 study in advanced NET (SMSUS 52) n 34 patients evaluable for toxicity (CTC v 3. 0) – Most frequent AE: mild aphthous ulceration – Grade 3/4 n Fatigue (n = 3) n Aphthous ulcers, diarrhea, rash (each n = 2) n Anemia, thrombocytopenia, neutropenia, leukocytosis, hyperglycemia, hypokalemia, hypophosphatemia, nausea, pruritus (each n = 1) Yao et al. ASCO 2006. Abstract 4042.

Conclusions (I) n Management with either biotherapy and chemotherapy can be guided by WHO classification in patients with malignant carcinoid. n Ki-67 proliferation index might be considered as an additional parameter for choosing between chemotherapy or biotherapy n Combination chemotherapy with Cisplatin, lederfolin, fluororuracil represents a valid therapeutic option in malignant carcinoid, having a good therapeutic index and favourable toxicity profile

Conclusions (II) n Among targeted therapies, RAD 001 may arrest NET growth by blocking downstream signaling through IGF -1 R, TSC 1/2, and m. TOR and, in combination with Octreotide LAR, may act synergistically to arrest NET growth and alleviate symptoms

Open Questions n A standard chemotherapy is still not in existence because a small number of patient cases and consequently a small number of randomized trials n What is the best treatment for Endocrine Pancreatic Tumors? n How can we select the best method of treatment for patients in the grey area (Ki-67 2 -15%) ?
 Fisiopatologia pancreas
Fisiopatologia pancreas Organi omologhi e analoghi
Organi omologhi e analoghi Organi omologhi e analoghi
Organi omologhi e analoghi Ode all amica risanata
Ode all amica risanata Walang sugat ni severino reyes
Walang sugat ni severino reyes Affluenti po
Affluenti po Nato a milano 1785
Nato a milano 1785 Centro siciliano di terapia della famiglia
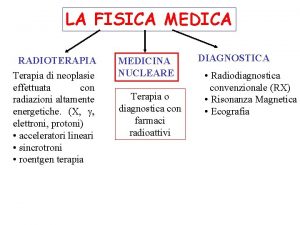
Centro siciliano di terapia della famiglia Fisica master
Fisica master Documentos para apelar licencia medica en compin
Documentos para apelar licencia medica en compin Modelo de prescrição médica
Modelo de prescrição médica Vocacion medicina
Vocacion medicina 1. ingeniera médica programadora periodista hijastra
1. ingeniera médica programadora periodista hijastra Academia nacional de educación médica
Academia nacional de educación médica Juan carlos arango barrientos
Juan carlos arango barrientos Ant tart materia medica
Ant tart materia medica Diceologia
Diceologia Receta cuantificada ejemplo
Receta cuantificada ejemplo Dott greggio gabriele
Dott greggio gabriele Art. 8 legge gelli
Art. 8 legge gelli Hepatotoxicos
Hepatotoxicos Icon
Icon Historia de la informatica medica
Historia de la informatica medica Gestin medica
Gestin medica Ricetta ripetibile formalismi
Ricetta ripetibile formalismi Specialista in fisica medica
Specialista in fisica medica Moto medica
Moto medica Ejemplos de auditoría médica
Ejemplos de auditoría médica Materia medica graphites
Materia medica graphites Domus medica
Domus medica El carmelo soyapango
El carmelo soyapango Anestetici locali
Anestetici locali Son los dos tipos de receta médica sicad
Son los dos tipos de receta médica sicad Avaliação médica dano corporal
Avaliação médica dano corporal Unife scuole di specializzazione
Unife scuole di specializzazione Dott losavio neurologo
Dott losavio neurologo Kalmia materia medica
Kalmia materia medica Uma barraca piramidal é sustentada por seis hastes
Uma barraca piramidal é sustentada por seis hastes Bear head
Bear head Academia nacional de educacion medica
Academia nacional de educacion medica Atlas medica cartella clinica
Atlas medica cartella clinica Que es rp en receta médica
Que es rp en receta médica Que es la lex artis
Que es la lex artis Materia medica reloaded
Materia medica reloaded Terapia dla nieśmiałych
Terapia dla nieśmiałych Terapia neuroacustica
Terapia neuroacustica Terapia mrt
Terapia mrt Acidi nucleici
Acidi nucleici Flunarizina acufeni
Flunarizina acufeni Terapia de pareja
Terapia de pareja Budesonide/formoterol
Budesonide/formoterol Anamnese terapia ocupacional idoso
Anamnese terapia ocupacional idoso Panipopitutarismo
Panipopitutarismo Deficiene
Deficiene