Leveraging Adaptive Implementation Strategies to Achieve Universal Coverage

















- Slides: 27

Leveraging Adaptive Implementation Strategies to Achieve Universal Coverage of Antiretroviral Treatment in Senegal Daouda Diouf Executive Director Enda Santé Dakar, Senegal 9 th IAS Conference on HIV Science Paris, France July 23, 2017

Outline • Background • Objective and Study Aims • Methods § HIV self testing (HIVST) § Biometric tracking system § Individualized case management • Challenges and Opportunities • Conclusions

Objectives • Assess the: § Feasibility § Fidelity § Cost-effectiveness Individualized case management to support sustained adherence to ART among individuals living with HIV who are not virally suppressed in Dakar and Ziguinchor, Senegal.

Specific Aims 1. Characterize the acceptability of HIV self-testing by people at risk of HIV infection in Senegal, and determine if the promotion of self-testing increases the number of newly diagnosed PLHIV in clinic settings. 2. Compare the effectiveness and durability of • • existing Standard of Care in Senegal versus individual case management programs to achieve sustained viral suppression among people living with HIV in Senegal. 3. Determine the cost-effectiveness of the universal treatment approach using the CM intervention. 4. Characterize the acceptability of iris scanning as a biometric follow up strategy to enhance the measurement of follow-up and retention of PLHIV receiving ART care.

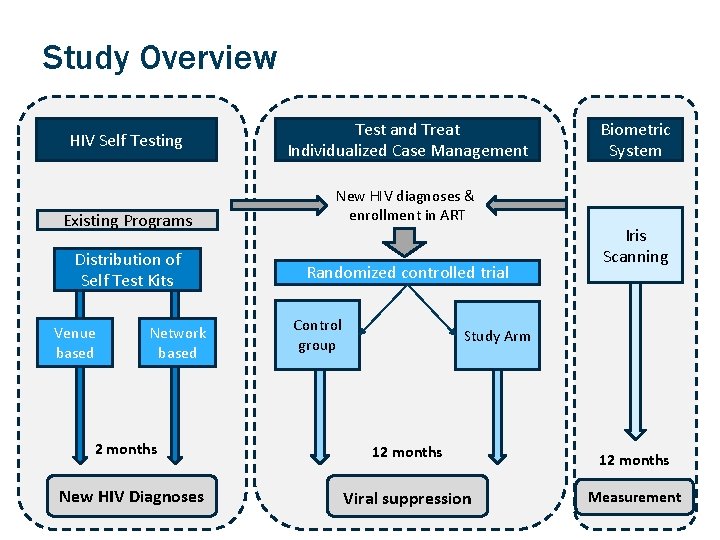
Study Overview HIV Self Testing Existing Programs Distribution of Self Test Kits Venue based Network based 2 months New HIV Diagnoses Test and Treat Individualized Case Management New HIV diagnoses & enrollment in ART Randomized controlled trial Control group Biometric System Iris Scanning Study Arm 12 months Viral suppression Measurement

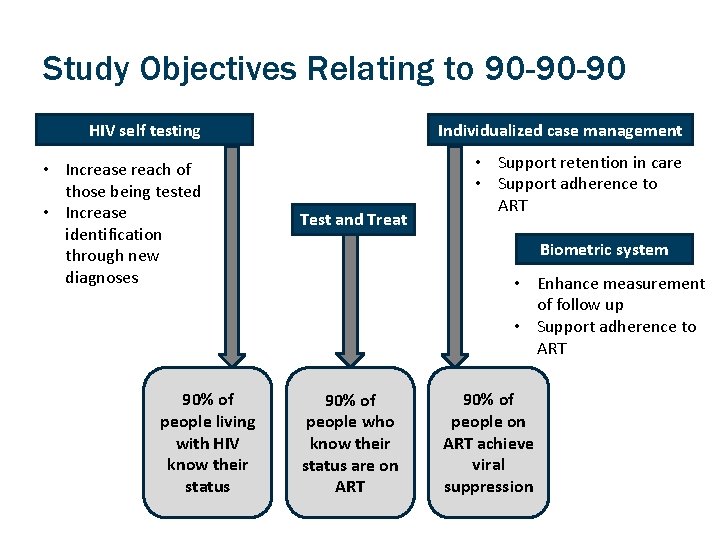
Study Objectives Relating to 90 -90 -90 HIV self testing Individualized case management • Increase reach of those being tested • Increase identification through new diagnoses • Support retention in care • Support adherence to ART 90% of people living with HIV know their status Test and Treat Biometric system • Enhance measurement of follow up • Support adherence to ART 90% of people who know their status are on ART 90% of people on ART achieve viral suppression

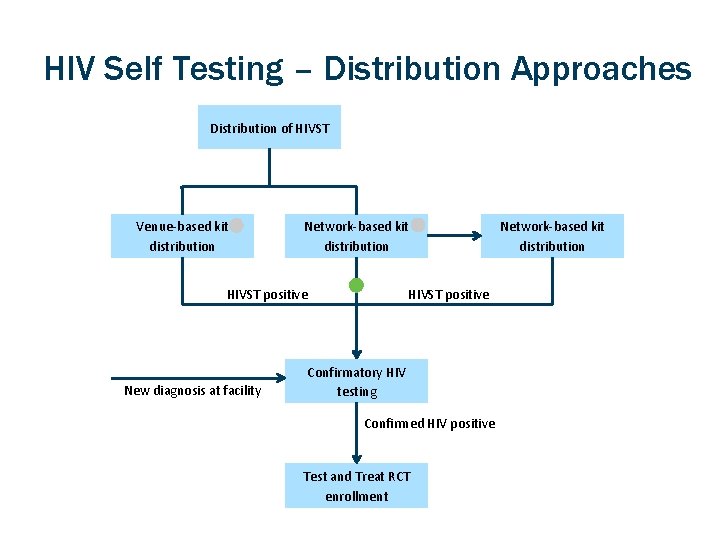
HIV Self Testing – Distribution Approaches Distribution of HIVST Venue-based kit distribution Network-based kit distribution HIVST positive New diagnosis at facility Confirmatory HIV testing Confirmed HIV positive Test and Treat RCT enrollment Network-based kit distribution

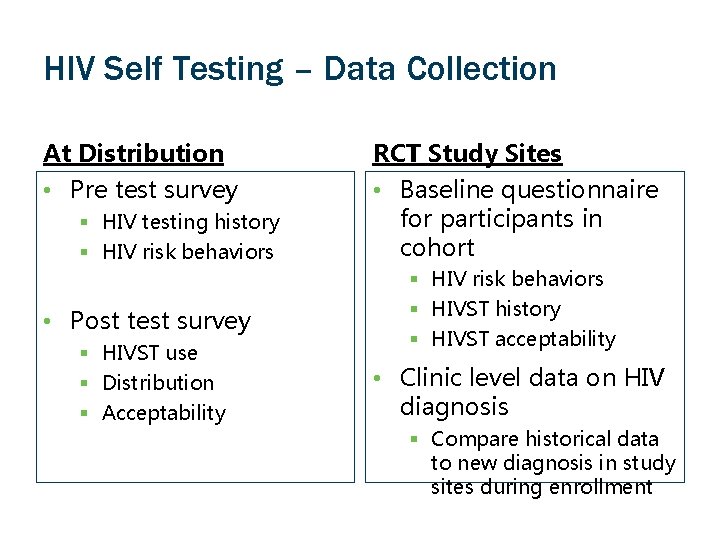
HIV Self Testing – Data Collection At Distribution RCT Study Sites • Pre test survey • Baseline questionnaire for participants in cohort § HIV testing history § HIV risk behaviors • Post test survey § HIVST use § Distribution § Acceptability § HIV risk behaviors § HIVST history § HIVST acceptability • Clinic level data on HIV diagnosis § Compare historical data to new diagnosis in study sites during enrollment

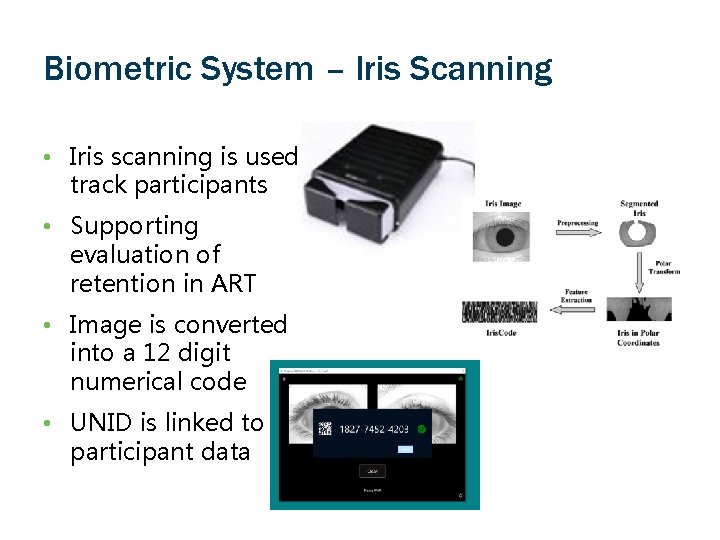
Biometric System – Iris Scanning • Iris scanning is used track participants • Supporting evaluation of retention in ART • Image is converted into a 12 digit numerical code • UNID is linked to participant data

Individualized Case Management – Study Design • Randomized control trial § Individualized case management + standard of care § Standard of care • Study sites § Government health facilities in Dakar and Ziguinchor • Sample size § 596 across all sites • Primary outcome: § Sustained viral suppression • <1, 000 copies/ml at 12 months after initial randomization.

Individualized Case Management – Study Arms • Intervention Arm: Case Management § Initial meeting between person living with HIV and case manager § Follow up meeting between case manager and participant § Biweekly automatic text messages sent to participant § Monthly phone calls from case manager § Face-to-face meetings between case manager and participant every 6 months • Control Arm § Standard of Care

HIV Self Testing – Challenges & Opportunities • Institutions are interested and want engagement § Feedback and approvals take time • Identified need to increase monitoring and data collection around HIVST distribution § Added pre/post test questionnaires § Aligning unique IDs across program • Perceived unreliable results

HIV Self Testing – Challenges & Opportunities • Government Engagement—Scientific committee • Integration in other national programs • Engagement of all stakeholders in developing strategies that intervene at all levels • Active in policy formulations— developing national strategic plan.

Biometric System – Challenges & Opportunities • New software, needs to be adapted to environment, update frequently • Internet connectivity § Wait time for code generation

Individualized Case Management – Challenges & Opportunities • Test and Treat in Senegal: guidelines vs implementation • Government facilities as study sites § Interest is high but approvals and agreements are slower § Limited resources • Case managers § Workload § Availability § Literacy • Concerns from clinics of enrolling § Patients with TB § Pregnant women • Randomization § First time that people have been randomized in government clinics

Conclusions • Significant interest in studying implementation strategies for Test and Treat • Difference between enacting guidelines and actually implementing § HIVST § Biometrics § Test and Treat • Government Buy In Vs Clinic Buy In § Clinic adherence to existing national guidelines

Acknowledgements • Study participants • Centre de Santé Dominique de Pikine, Centre de Santé de Ziguinchor • DLSI • CNLS • USAID • SOAR/Population Council • Johns Hopkins University — Key Populations Program

Thank You Project SOAR (Cooperative Agreement AID-OAA-A-14 -00060) is made possible by the generous support of the American people through the President’s Emergency Plan for AIDS Relief (PEFPAR) and United States Agency for International Development (USAID). The contents of this presentation are the sole responsibility of Project SOAR and Population Council and do not necessarily reflect the views of USAID or the United States Government. Through operations research, Project SOAR will determine how best to address challenges and gaps that remain in the delivery of HIV and AIDS care and support, treatment, and prevention services. Project SOAR will produce a large, multifaceted body of high-quality evidence to guide the planning and implementation of HIV and AIDS programs and policies. Led by the Population Council, Project SOAR is implemented in collaboration with Avenir Health, Elizabeth Glaser Pediatric AIDS Foundation, Johns Hopkins University, Palladium, and The University of North Carolina.

Appendices 1. Evidence Supporting Universal Coverage of ART 2. ANRS 12249 Universal Treatment Trial 3. ANRS 12249 Tas. P: HIV incidence comparison 4. HPTN 071 (Pop. ART) Preliminary Data 5. HIV Self Testing – Distribution Approaches 6. HIV Self Testing – Supervision 7. Individualized Case Management –

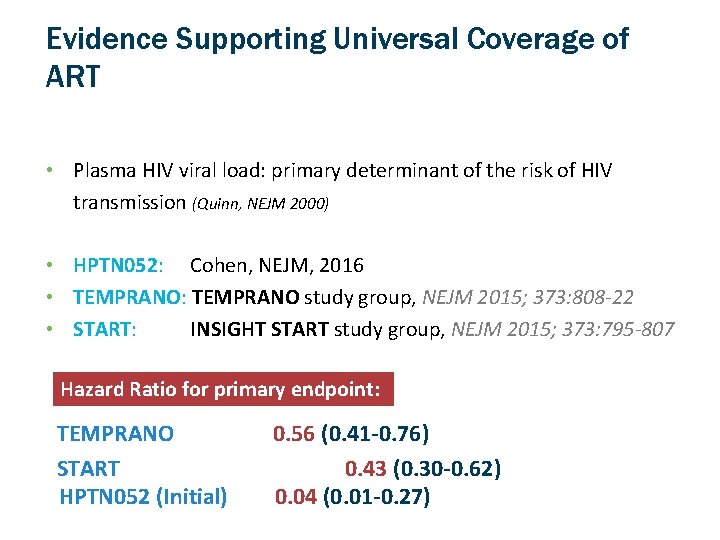
Evidence Supporting Universal Coverage of ART • Plasma HIV viral load: primary determinant of the risk of HIV transmission (Quinn, NEJM 2000) • HPTN 052: Cohen, NEJM, 2016 • TEMPRANO: TEMPRANO study group, NEJM 2015; 373: 808 -22 • START: INSIGHT START study group, NEJM 2015; 373: 795 -807 Hazard Ratio for primary endpoint: TEMPRANO START HPTN 052 (Initial) 0. 56 (0. 41 -0. 76) 0. 43 (0. 30 -0. 62) 0. 04 (0. 01 -0. 27)

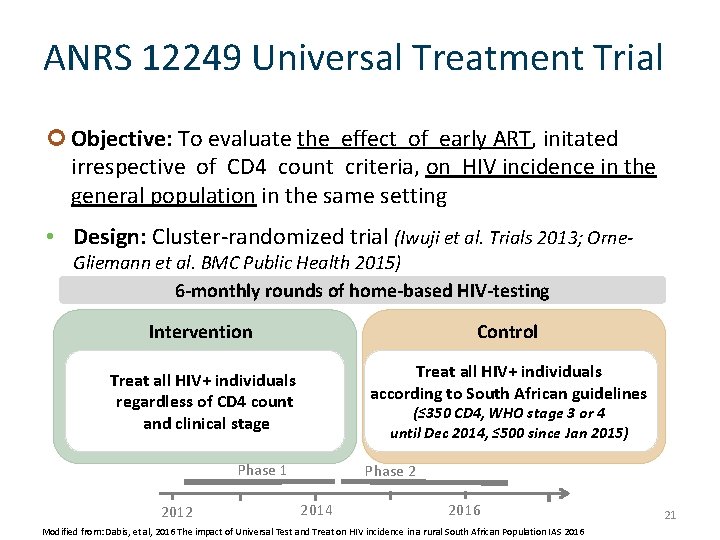
ANRS 12249 Universal Treatment Trial Objective: To evaluate the effect of early ART, initated irrespective of CD 4 count criteria, on HIV incidence in the general population in the same setting • Design: Cluster-randomized trial (Iwuji et al. Trials 2013; Orne. Gliemann et al. BMC Public Health 2015) 6 -monthly rounds of home-based HIV-testing Intervention Control Treat all HIV+ individuals regardless of CD 4 count and clinical stage Treat all HIV+ individuals according to South African guidelines (≤ 350 CD 4, WHO stage 3 or 4 until Dec 2014, ≤ 500 since Jan 2015) Phase 1 2012 Phase 2 2014 2016 Modified from: Dabis, et al, 2016 The impact of Universal Test and Treat on HIV incidence in a rural South African Population IAS 2016 21

ANRS 12249 Universal Treatment Trial Intervention Control 91% 52% At month 6 93% 92% At month 12 95% Estimated ART coverage* (as of 1 st January 2016) 45% 43% ART coverage improvement since baseline +14 +7 ART initiation within 3 months in Tas. P clinics among patients not on ART at first Tas. P clinic visit Viral load <400 copies/ml among patients not on ART at first Tas. P clinic visit * Estimated from Tas. P + Department of Health data 22 Modified from: Dabis, et al, 2016 The impact of Universal Test and Treat on HIV incidence in a rural South African Population IAS 2016

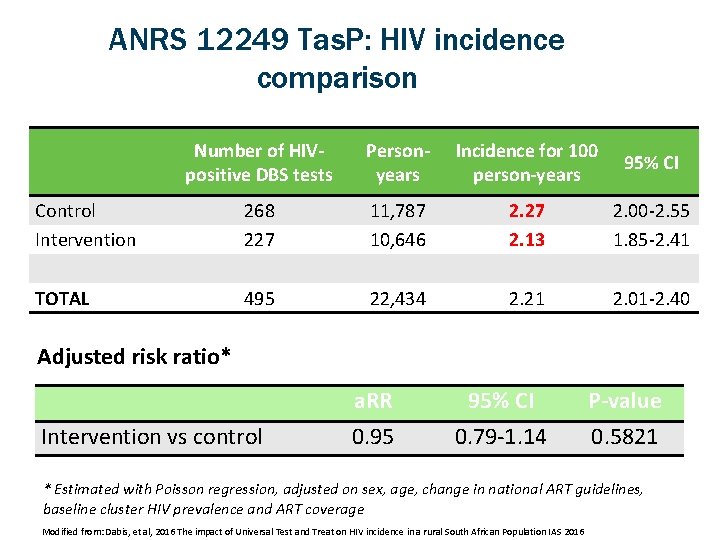
ANRS 12249 Tas. P: HIV incidence comparison Number of HIVpositive DBS tests Personyears Incidence for 100 person-years 95% CI Control Intervention 268 227 11, 787 10, 646 2. 27 2. 13 2. 00 -2. 55 1. 85 -2. 41 TOTAL 495 22, 434 2. 21 2. 01 -2. 40 Adjusted risk ratio* Intervention vs control a. RR 0. 95 95% CI 0. 79 -1. 14 P-value 0. 5821 * Estimated with Poisson regression, adjusted on sex, age, change in national ART guidelines, baseline cluster HIV prevalence and ART coverage Modified from: Dabis, et al, 2016 The impact of Universal Test and Treat on HIV incidence in a rural South African Population IAS 2016

HPTN 071 (Pop. ART) Preliminary Data • “Acceptance of HIV testing among those consenting to the intervention was high, although linkage to care and ART initiation took longer than expected”. § What about adherence? Hayes, et al. May, 2017 http: //journals. plos. org/plosmedicine/article? id=10. 1371/journal. pmed. 1002292

HIV Self Testing – Distribution Approaches KEY Peer educator trained in distribution methods Participant Supervised distribution HIVST Unsupervised distribution HIVST Referral Participant consented and completes survey Venue-based distribution Network-based Distribution

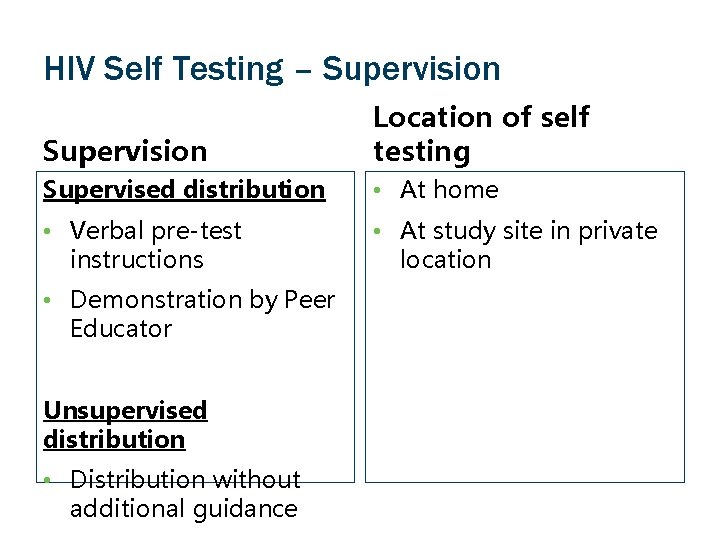
HIV Self Testing – Supervision Location of self testing Supervised distribution • At home • Verbal pre-test instructions • At study site in private location • Demonstration by Peer Educator Unsupervised distribution • Distribution without additional guidance

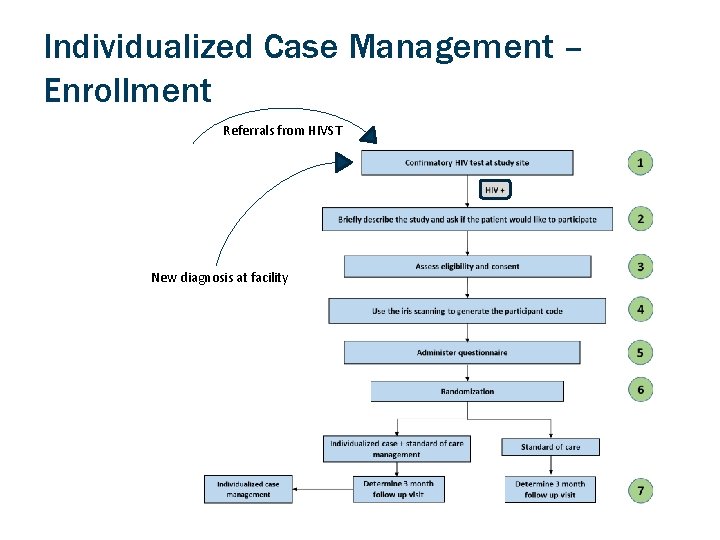
Individualized Case Management – Enrollment Referrals from HIVST New diagnosis at facility