Lets Review orbitals defined by their shape orientation
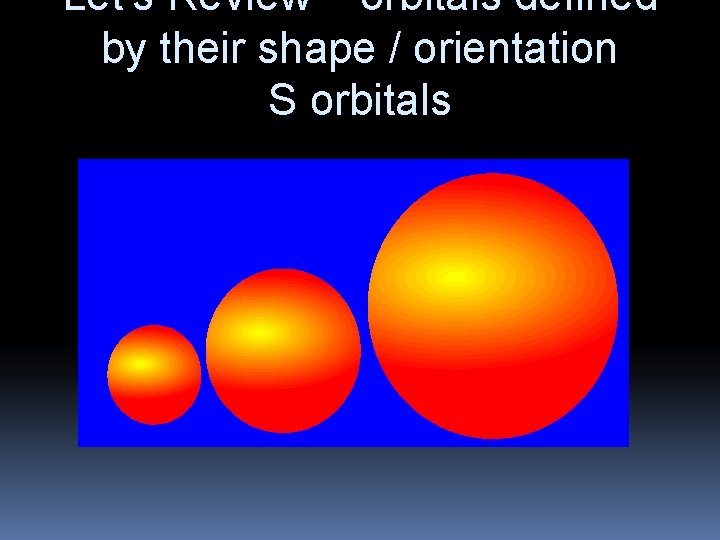
Let’s Review – orbitals defined by their shape / orientation S orbitals
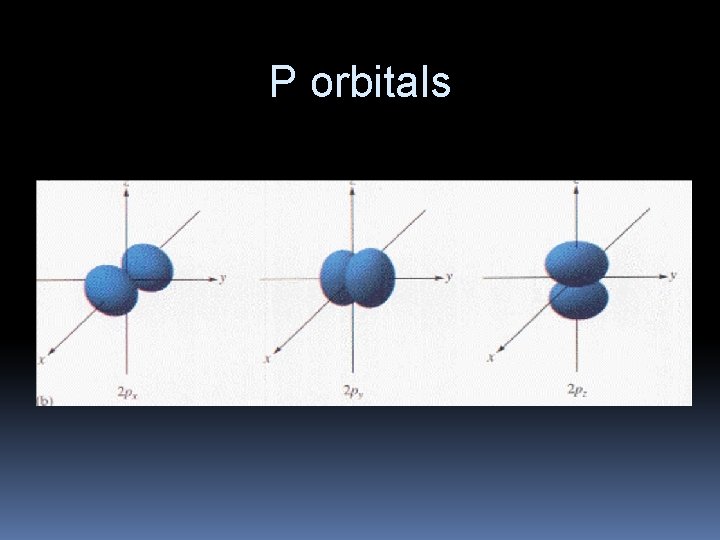
P orbitals
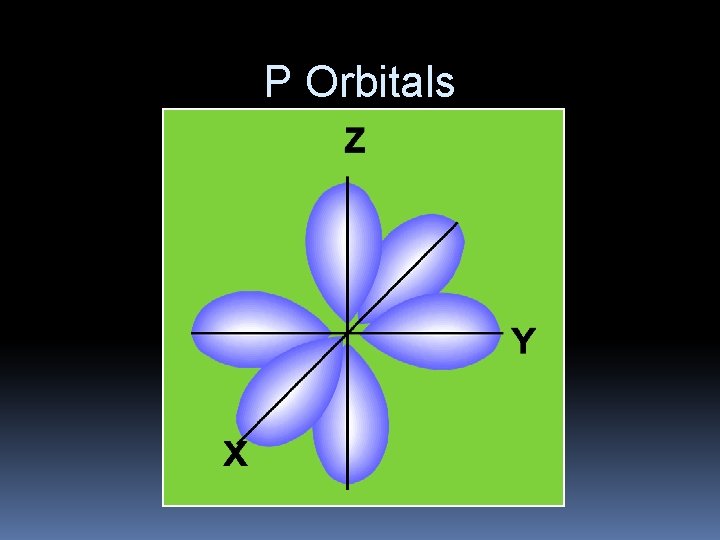
P Orbitals
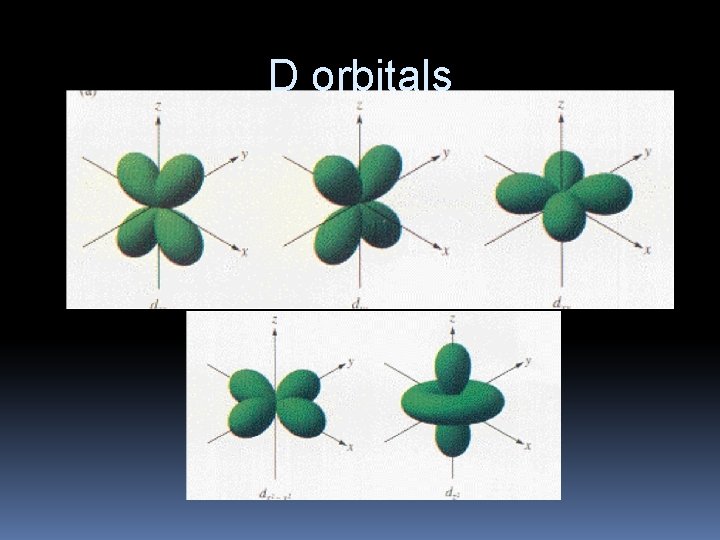
D orbitals
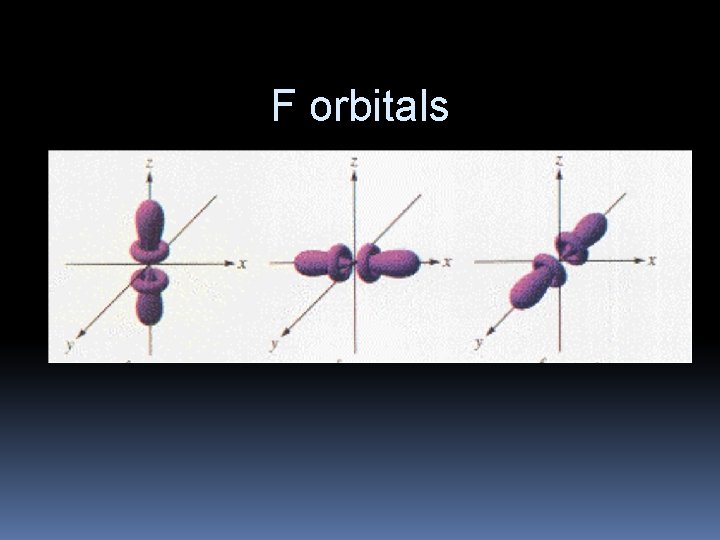
F orbitals
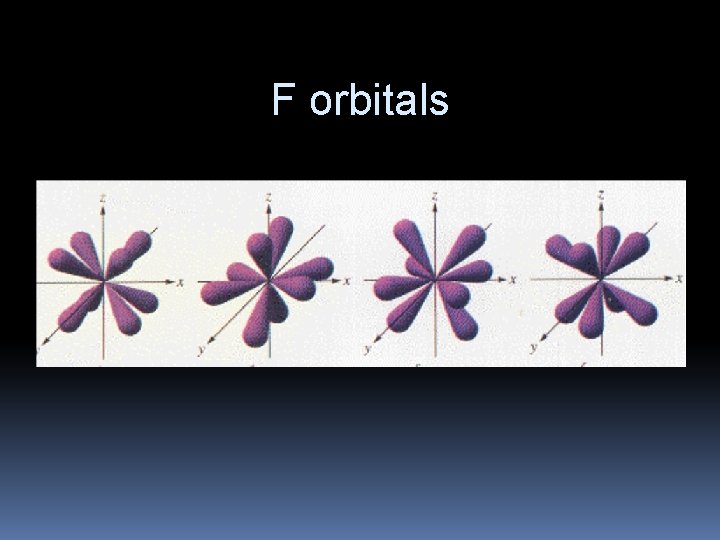
F orbitals

Quantum numbers Magnetic quantum number (m l) integer values between - l and + l tells direction in each shape. Electron spin quantum number (m s) Can have 2 values. either +1/2 or -1/2
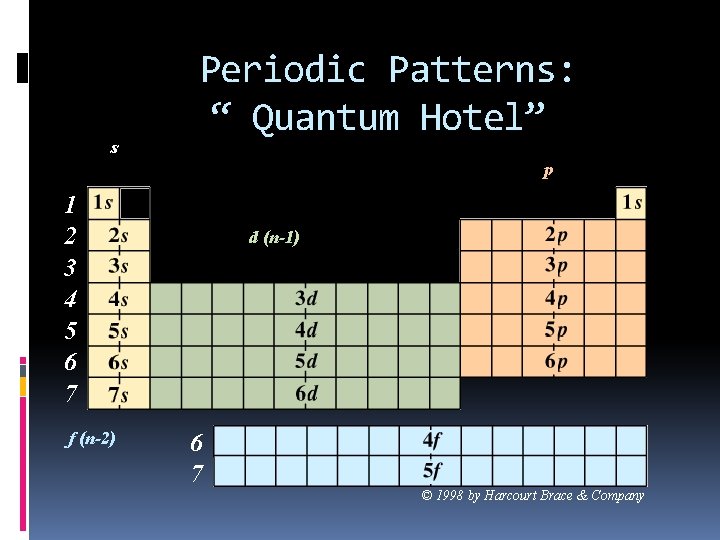
s Periodic Patterns: “ Quantum Hotel” p 1 2 3 4 5 6 7 f (n-2) d (n-1) 6 7 © 1998 by Harcourt Brace & Company
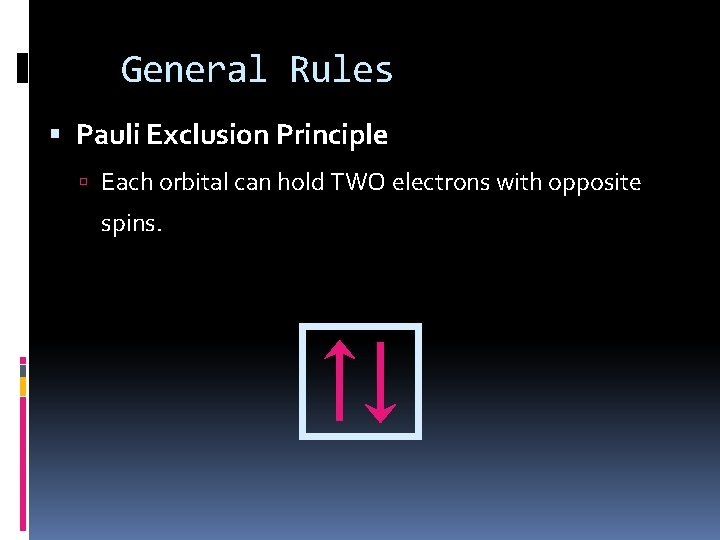
General Rules Pauli Exclusion Principle Each orbital can hold TWO electrons with opposite spins.
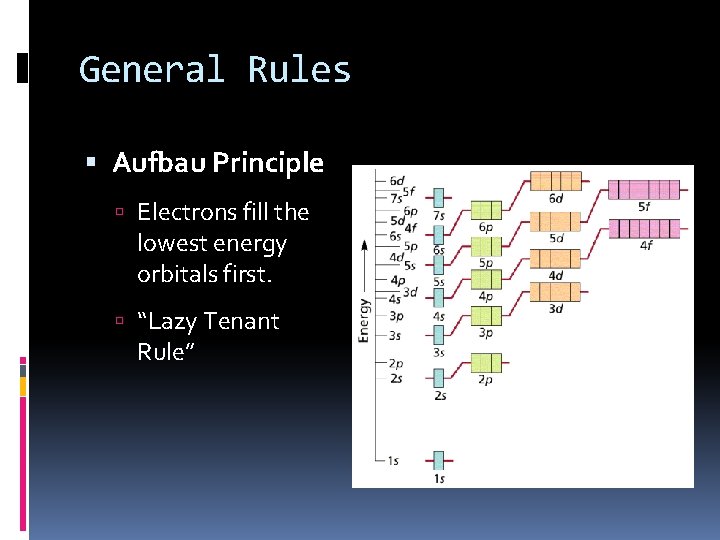
General Rules Aufbau Principle Electrons fill the lowest energy orbitals first. “Lazy Tenant Rule”

General Rules Hund’s Rule Within a sublevel, place one e- per orbital before pairing them. “Empty Bus Seat Rule” WRONG RIGHT
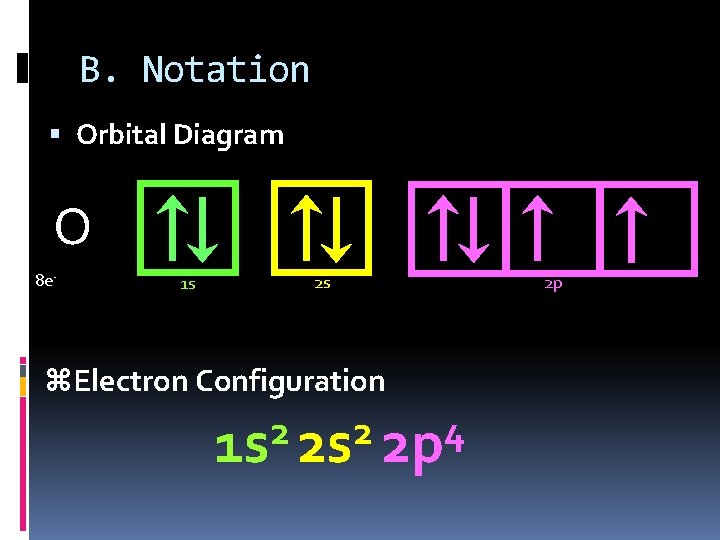
B. Notation Orbital Diagram O 8 e- 1 s 2 s z. Electron Configuration 2 2 4 1 s 2 s 2 p 2 p
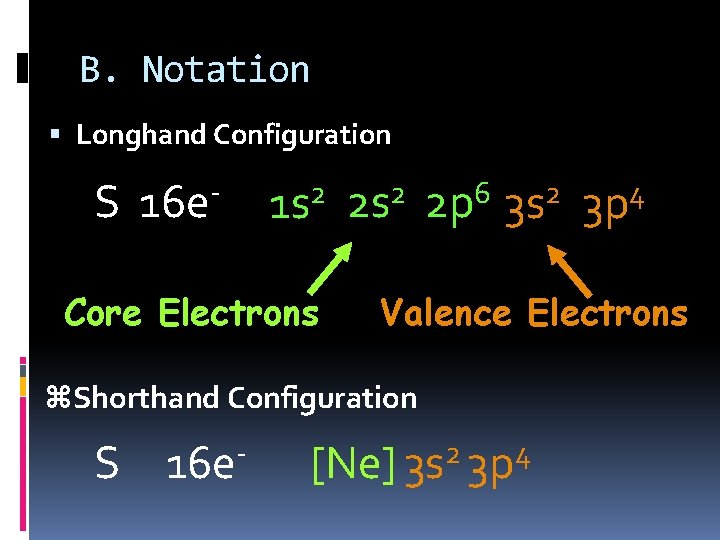
B. Notation Longhand Configuration S 16 e 2 1 s Core Electrons 2 2 s 6 2 p 2 3 s Valence Electrons z. Shorthand Configuration S 16 e 4 3 p 2 4 [Ne] 3 s 3 p
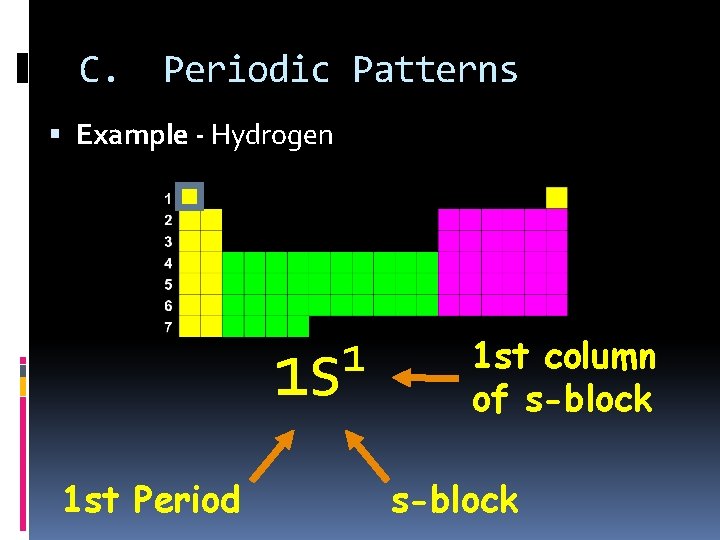
C. Periodic Patterns Example - Hydrogen 1 1 s 1 st Period 1 st column of s-block
![Nobel Gas Notation Example - Germanium 2 [Ar] 4 s 10 3 d 2 Nobel Gas Notation Example - Germanium 2 [Ar] 4 s 10 3 d 2](http://slidetodoc.com/presentation_image_h2/212aa01858f5176b41e7fdeff9afa590/image-15.jpg)
Nobel Gas Notation Example - Germanium 2 [Ar] 4 s 10 3 d 2 4 p
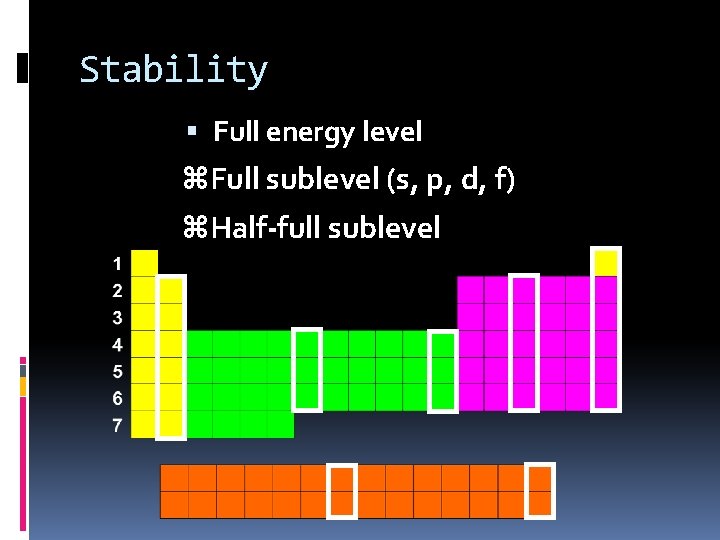
Stability Full energy level z. Full sublevel (s, p, d, f) z. Half-full sublevel
![Stability Electron Configuration Exceptions Copper EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 9 Stability Electron Configuration Exceptions Copper EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 9](http://slidetodoc.com/presentation_image_h2/212aa01858f5176b41e7fdeff9afa590/image-17.jpg)
Stability Electron Configuration Exceptions Copper EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 9 [Ar] 4 s 1 3 d 10 Copper gains stability with a full d-sublevel.
![Stability Electron Configuration Exceptions Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 4 Stability Electron Configuration Exceptions Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 4](http://slidetodoc.com/presentation_image_h2/212aa01858f5176b41e7fdeff9afa590/image-18.jpg)
Stability Electron Configuration Exceptions Chromium EXPECT: ACTUALLY: [Ar] 4 s 2 3 d 4 [Ar] 4 s 1 3 d 5 Chromium gains stability with a half-full dsublevel.
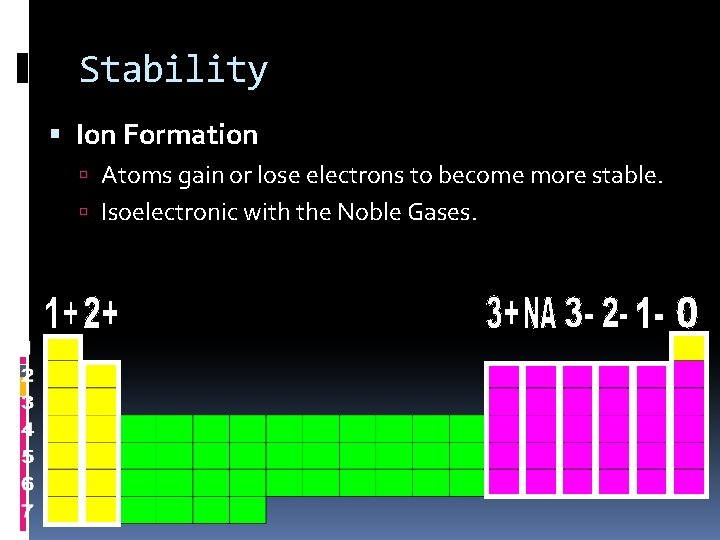
Stability Ion Formation Atoms gain or lose electrons to become more stable. Isoelectronic with the Noble Gases.

Which is it? Is energy a wave like light, or a particle? BOTH! Light behaves as a wave under certain conditions, and as a stream of particles under others. Concept is called the Wave -Particle duality. What about the other way, is matter a wave? Yes
- Slides: 20