Lesson Starter Can you define a chemical reaction







- Slides: 16

Lesson Starter • Can you define a ‘chemical reaction’? • What do the following words mean: – ENDOTHERMIC – EXOTHERMIC

• Can you define a ‘chemical reaction’? • A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. • What do the following words mean: – ENDOTHERMIC – takes in heat – EXOTHERMIC – gives out heat

Rates of Reaction 5. 7 Investigate the effect of temperature, concentration and surface area of a solid on the rate of a reaction such as hydrochloric acid and marble chips 5. 8 Recall that the rates of chemical reactions vary explosive reactions to very slow reactions 5. 9 Describe the effect of changes in temperature, concentration surface area of a solid on the rate of reaction 5. 10 Describe how reactions can occur when particles collide and explain how rates of reaction are increased by increasing the frequency and/or energy of collisions 5. 11 Demonstrate an understanding that not all collisions lead to a reaction, especially if particles collide with low energy

Dissolving jelly Your task: • Dissolve an entire block of jelly in the fastest time possible, using ONLY the equipment provided. The RULES: • You must stick to your own group area • You must work safely (no silly behaviour) • ALL jelly must be dissolved. • You must produce a clear written summary of what you did to dissolve all the jelly. This can be in any form you wish – mind map, list, paragraph.

Plenary • What did you find out from your experiment? • What increased the rate at which the jelly dissolved? • Was there more than one thing?

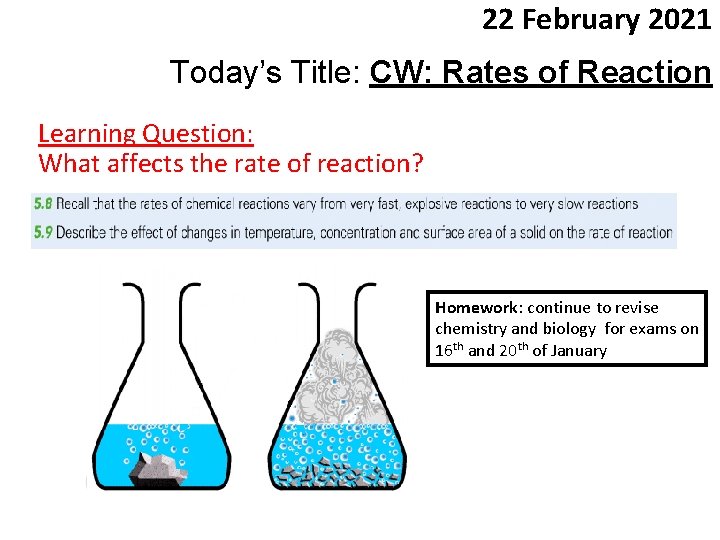
22 February 2021 Today’s Title: CW: Rates of Reaction Learning Question: What affects the rate of reaction? Homework: continue to revise chemistry and biology for exams on 16 th and 20 th of January

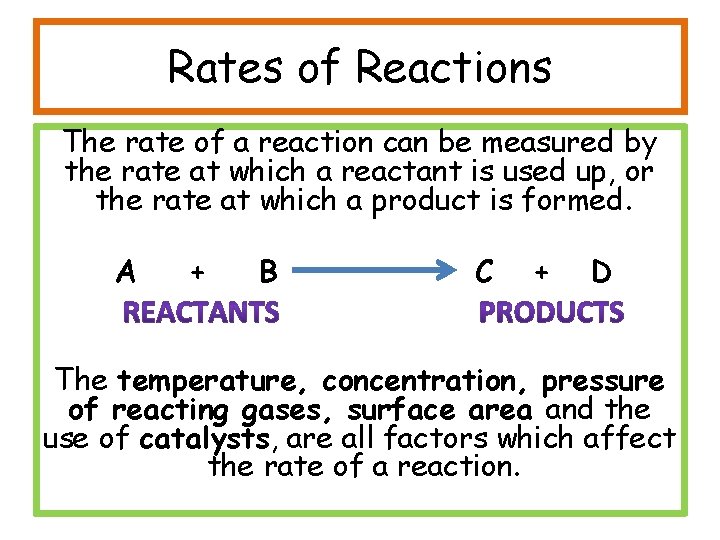
Rates of Reactions The rate of a reaction can be measured by the rate at which a reactant is used up, or the rate at which a product is formed. A + B C + D The temperature, concentration, pressure of reacting gases, surface area and the use of catalysts, are all factors which affect the rate of a reaction.

Collision Theory Chemical reactions can only happen if reactant particles collide with enough energy. The more frequently particles collide, and the greater the proportion of collisions with enough energy, the greater the rate of reaction.

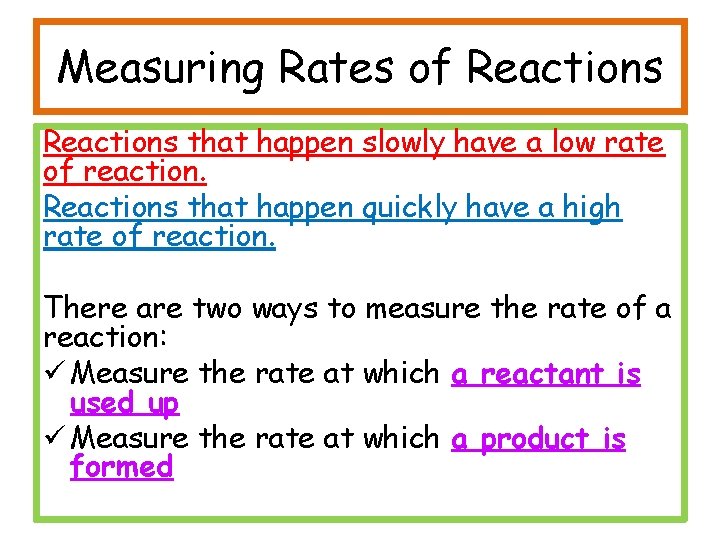
Measuring Rates of Reactions that happen slowly have a low rate of reaction. Reactions that happen quickly have a high rate of reaction. There are two ways to measure the rate of a reaction: ü Measure the rate at which a reactant is used up ü Measure the rate at which a product is formed

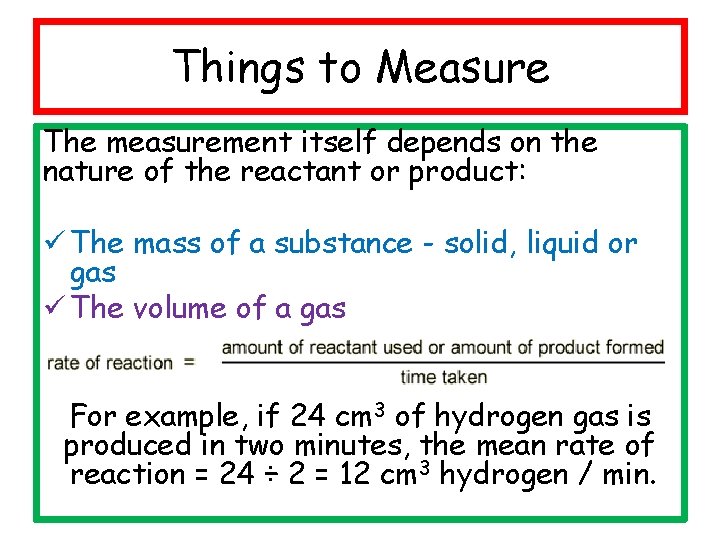
Things to Measure The measurement itself depends on the nature of the reactant or product: ü The mass of a substance - solid, liquid or gas ü The volume of a gas For example, if 24 cm 3 of hydrogen gas is produced in two minutes, the mean rate of reaction = 24 ÷ 2 = 12 cm 3 hydrogen / min.

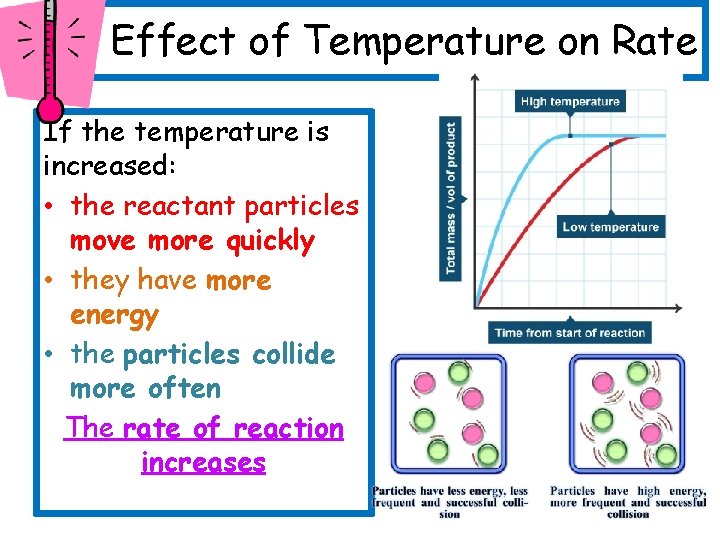
Effect of Temperature on Rate If the temperature is increased: • the reactant particles move more quickly • they have more energy • the particles collide more often The rate of reaction increases

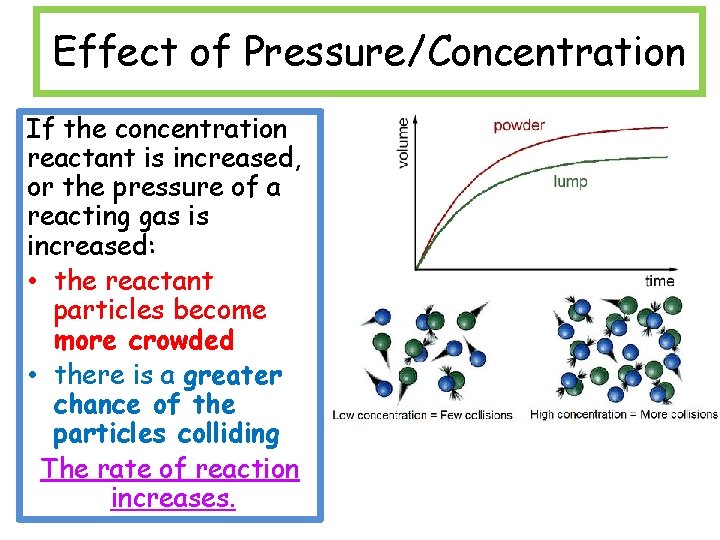
Effect of Pressure/Concentration If the concentration reactant is increased, or the pressure of a reacting gas is increased: • the reactant particles become more crowded • there is a greater chance of the particles colliding The rate of reaction increases.

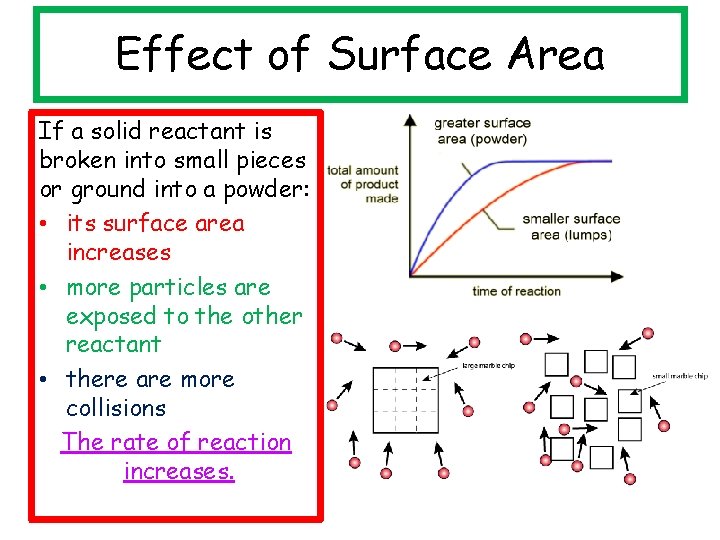
Effect of Surface Area If a solid reactant is broken into small pieces or ground into a powder: • its surface area increases • more particles are exposed to the other reactant • there are more collisions The rate of reaction increases.

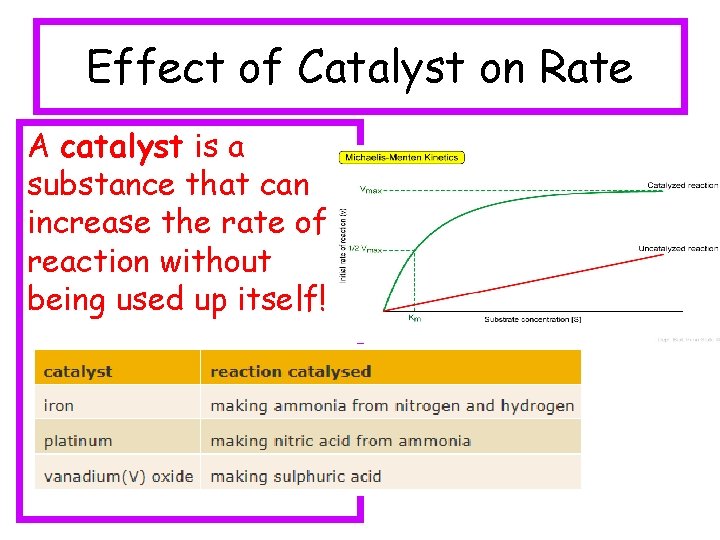
Effect of Catalyst on Rate A catalyst is a substance that can increase the rate of a reaction without being used up itself!

Watch this • Click here to watch the video that summarises rates of reaction. (Ted Ed) • Click here to watch another video that summarises rates of reaction and the collision theory. (BBC Learning Zone)

Do this • Collect a textbook at complete questions 1 – 5 on pages 170 -171. • It’s really important that you do this to consolidate the work you have just done