Lesson Essential Question How do molecules interact with




- Slides: 13

Lesson Essential Question How do molecules interact with each other?

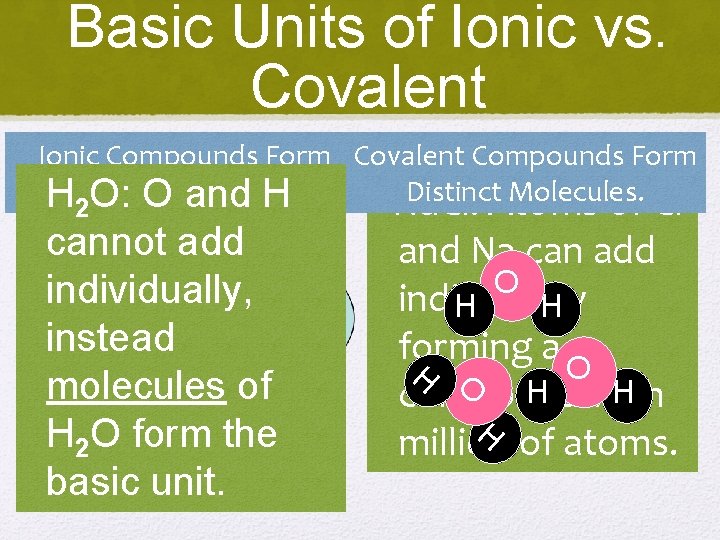
Basic Units of Ionic vs. Covalent Ionic Compounds Form Covalent Compounds Form Units. Distinct. Atoms Molecules. H Repeating O: O and H Na. Cl: of Cl 2 cannot add individually, instead molecules of H 2 O form the basic unit. and Na can add O individually H H forming a O H with H compound H million of atoms.

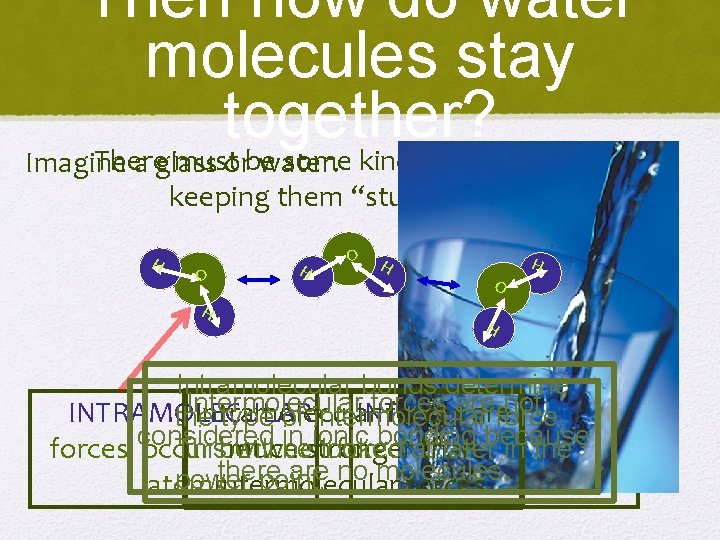
Then how do water molecules stay together? There must be some kind of attractive force Imagine a glass or water. keeping them “stuck” together. H O H O H H Intramolecular bonds determine Intermolecular forces are not INTRAMOLECULAR Intramolecular INTERMOLECULAR forces are the type of intermolecular force. in stronger ionic bonding Thisbetween will be looked atthan laterbecause in the forces considered occur much forces occur between there are no molecules. power point. atoms. Intermolecular forces. molecules.

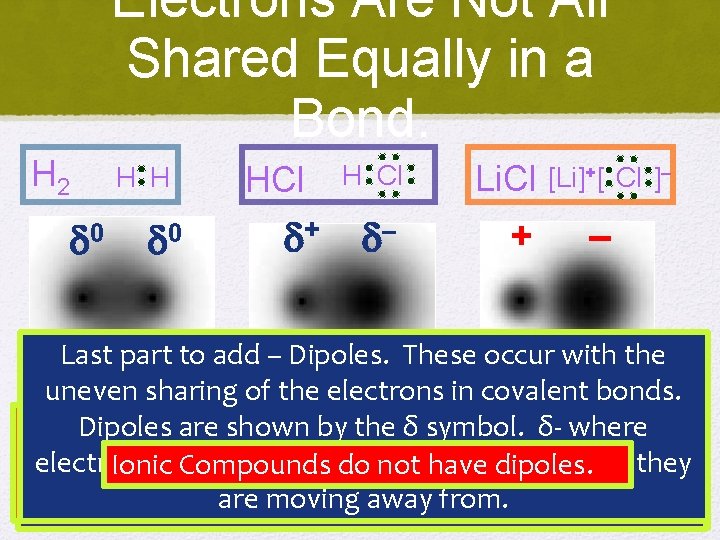
Electrons Are Not All Shared Equally in a Bond. H 2 H H 0 0 HCl + H Cl Li. Cl – + [Li]+ [ Cl ]– – Last part to add – Dipoles. These occur with the Covalent Ionic (non-polar) uneven sharing Polar of the Covalent electrons in covalent bonds. Dipoles are shown by the δof symbol. δ- has where Li Recall has that an electronegativity is 1. 0 “a and number Cl has an H has an electronegativity of 2. 1. Since itthat is H has an electronegativity of 2. 1 and Cl an You can calculate the nature of the bond by To figure out which type of bond each compound electrons are more concentrated and δ+ where they electronegativity describes the relative of 3. 0. ability The ΔEN of an isis 2. 1 3. 0 atom, – 2. 1 1. 0 when == 2. 0. Ionic Compounds do not have dipoles. connected to another H the ΔEN is – = 0. This electronegativity of 3. 0. The ΔEN 3. 0 – 2. 1 0. 9. subtracting (ΔEN) the two electronegativities. has you need to look at electronegativity. are moving away from. bonded, This it an ionic electrons” bond. makes itmakes a non-polar covalent This makes ittoaattract polar

Overview Basically: a EN below 0. 5 = covalent (non-polar) 0. 5 - 1. 7 = polar covalent above 1. 7 = ionic

Try These Determine the EN and bond type for these: 1. HCl 2. Cr. O 3. Br 2 4. H 2 O 5. CH 4 6. KCl

Answer’s HCl: 3. 0 – 2. 1 = 0. 9 polar covalent Cr. O: 3. 5 – 1. 6 = 1. 9 ionic Br 2: 2. 8 – 2. 8 = 0 covalent H 2 O: 3. 5 – 2. 1 = 1. 4 polar covalent CH 4: 2. 5 – 2. 1 = 0. 4 covalent KCl: 3. 0 – 0. 8 =2. 2 ionic

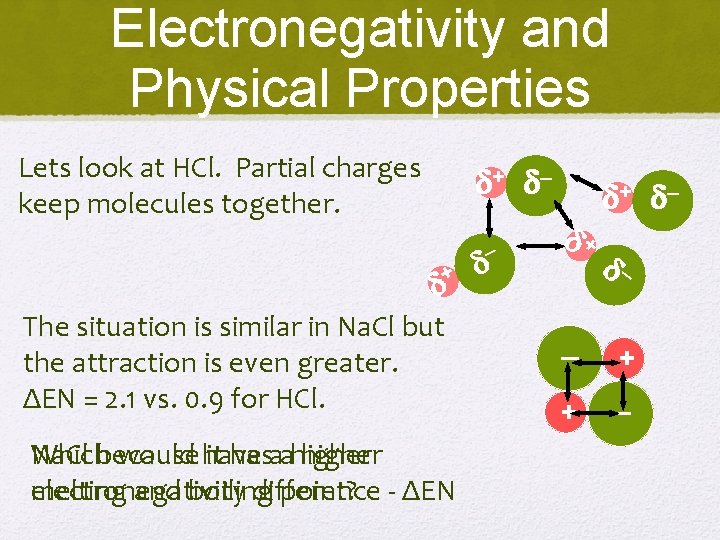
Electronegativity and Physical Properties Lets look at HCl. Partial charges keep molecules together. + – + The situation is similar in Na. Cl but the attraction is even greater. ΔEN = 2. 1 vs. 0. 9 for HCl. Na. Cl Whichbecause would have it hasaahigher electronegativity melting and boiling difference point? - ΔEN – + + – – – + + –

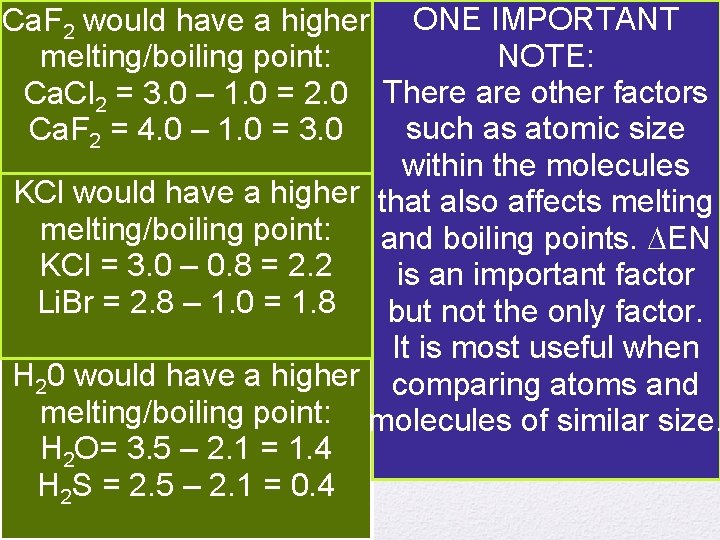
Which has the higher Boiling Point? Ca. F 2 would have a higher ONE IMPORTANT NOTE: melting/boiling point: Ca. Cl 2 = 3. 0 – 1. 0 = 2. 0 There are other factors such as atomic size Ca. F 2 = 4. 0 – 1. 0 = 3. 0 within the molecules KCl 1. would have a higher that also affects melting Ca. Cl , Ca. F 2 2 melting/boiling point: and boiling points. EN 2. =KCl, KCl 3. 0 –Li. Br 0. 8 = 2. 2 is an important factor Li. Br = 2. 8 – 1. 0 = 1. 8 but not the only factor. 3. H 2 O, H 2 S It is most useful when H 20 would have a higher comparing atoms and melting/boiling point: molecules of similar size. H 2 O= 3. 5 – 2. 1 = 1. 4 H 2 S = 2. 5 – 2. 1 = 0. 4

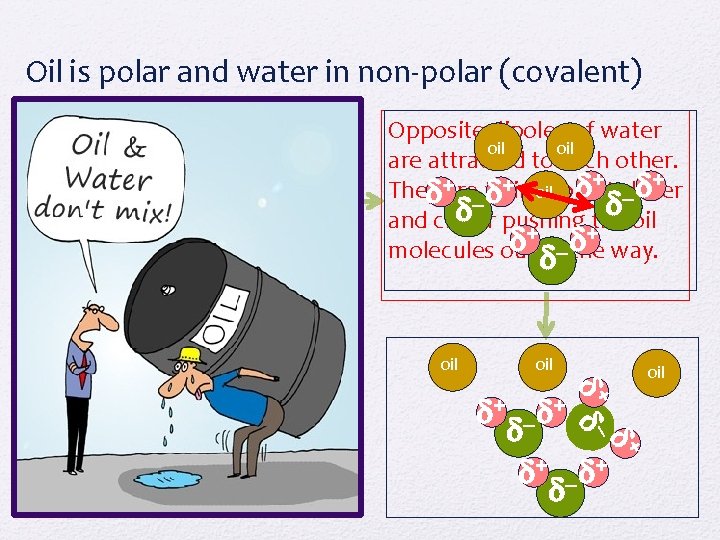
Oil and Water Why do oil and water never mix?

Oil is polar and water in non-polar (covalent) + – + oil oil + – + Opposite dipoles of water oil are attracted to each other. + + They are trying to get closer oil – – pushing the oil and closer + + molecules out of– the way. oil oil + + –

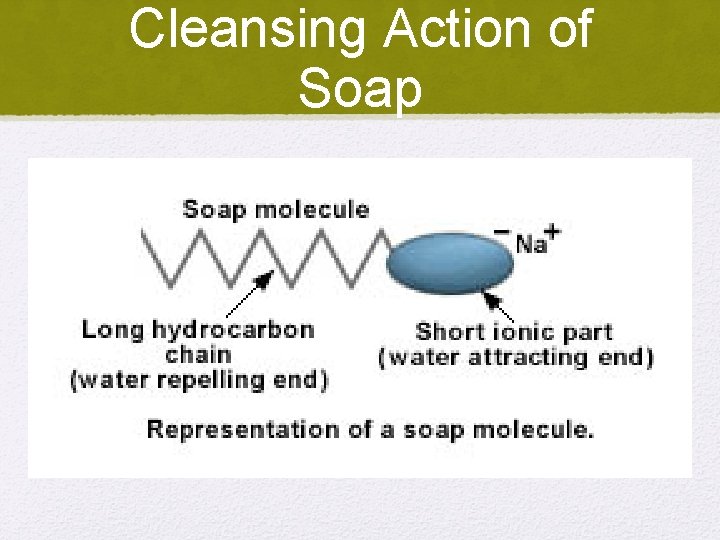
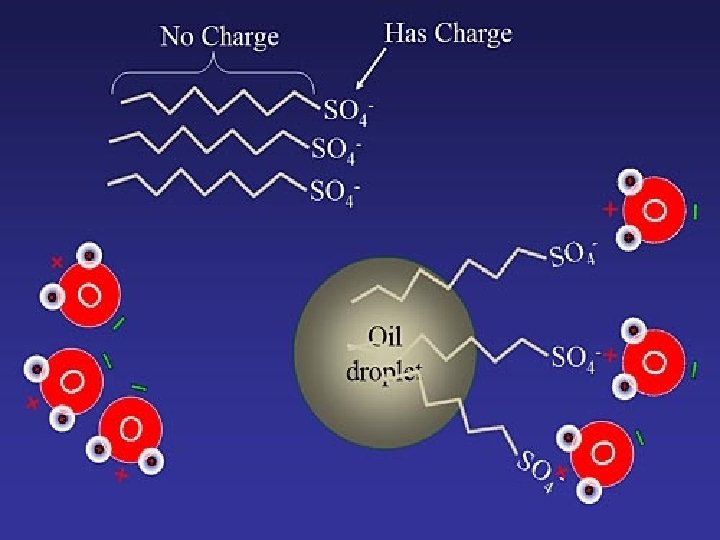
Cleansing Action of Soap
