Lesson 7 Examining and Grouping Elements You will





- Slides: 13

Lesson 7 Examining and Grouping Elements You will study the characteristics of 25 element samples and look at how they are grouped on the Periodic Table. Getting Started : Name some characteristic properties.

Lesson 7 What properties of elements can be used in grouping elements?

Lesson 7 If we examine several elements then we can group them in a meaningful way because we can use their characteristic properties.

Lesson 7

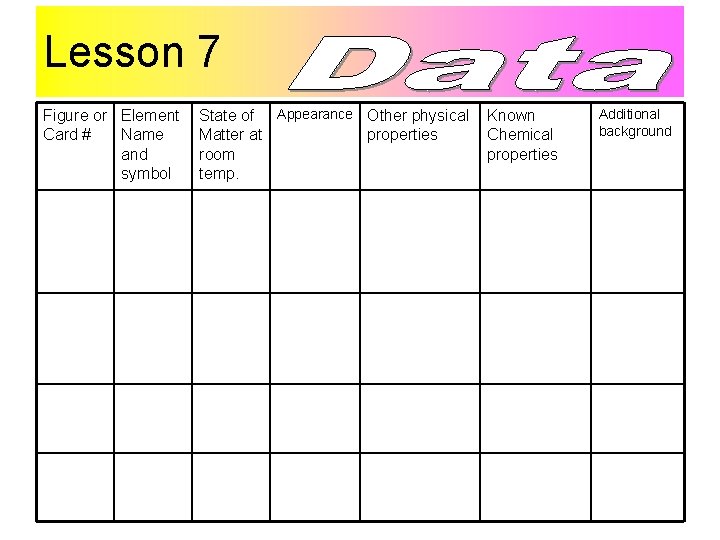
Lesson 7 Figure or Element Card # Name and symbol State of Appearance Other physical Matter at properties room temp. Known Chemical properties Additional background

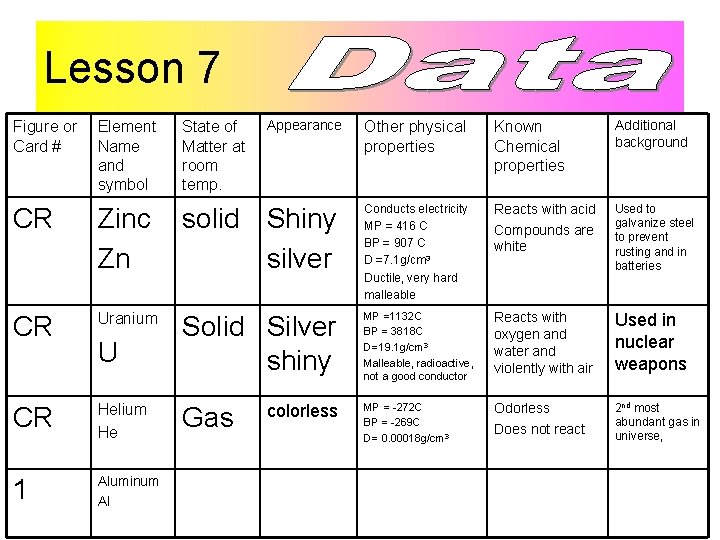
Lesson 7 Figure or Card # Element Name and symbol CR Zinc Zn CR Uranium CR Helium He 1 Aluminum Al U Other physical properties Known Chemical properties Additional background solid Shiny silver Conducts electricity MP = 416 C BP = 907 C D =7. 1 g/cm 3 Ductile, very hard malleable Reacts with acid Compounds are white Used to galvanize steel to prevent rusting and in batteries Solid Silver shiny MP =1132 C BP = 3818 C D=19. 1 g/cm 3 Malleable, radioactive, not a good conductor Reacts with oxygen and water and violently with air Used in nuclear weapons MP = -272 C BP = -269 C D= 0. 00018 g/cm 3 Odorless Does not react 2 nd most abundant gas in universe, State of Matter at room temp. Gas Appearance colorless

Lesson 7 With your lab group: Determine 5 separate categories for the elements studied You must be able to explain your rationale for the groups. Place all elements in one of the 5 groups. Write your categories with the elements in your journal Record your group’s idea on the large construction paper provided.

Lesson 7 • Elements are grouped according to similar chemical and physical properties. • The Periodic Table is used in predicting the chemical and physical properties of elements. • Each element can be identified by its characteristic properties.

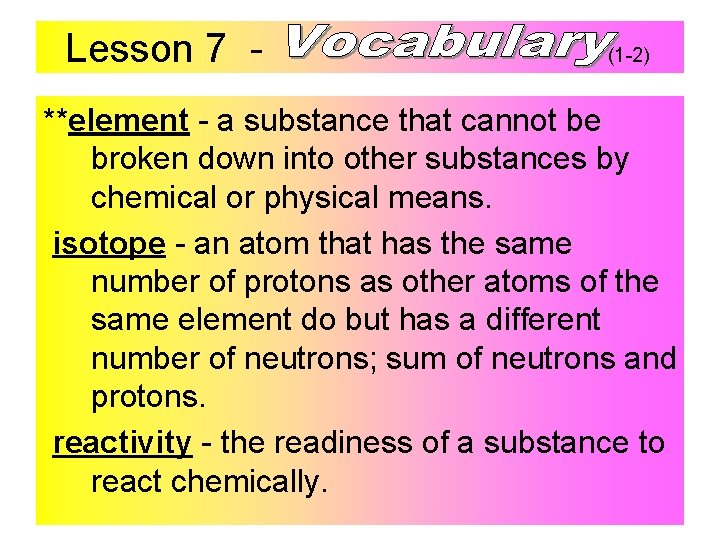
Lesson 7 - (1 -2) **element - a substance that cannot be broken down into other substances by chemical or physical means. isotope - an atom that has the same number of protons as other atoms of the same element do but has a different number of neutrons; sum of neutrons and protons. reactivity - the readiness of a substance to react chemically.

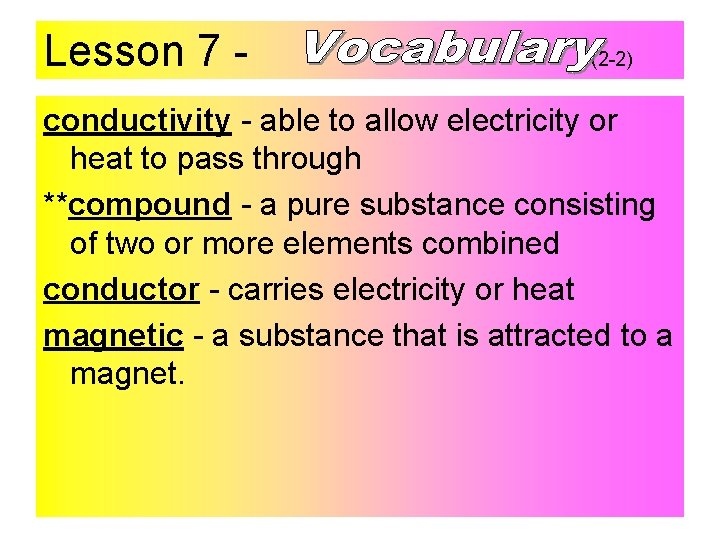
Lesson 7 - (2 -2) conductivity - able to allow electricity or heat to pass through **compound - a pure substance consisting of two or more elements combined conductor - carries electricity or heat magnetic - a substance that is attracted to a magnet.

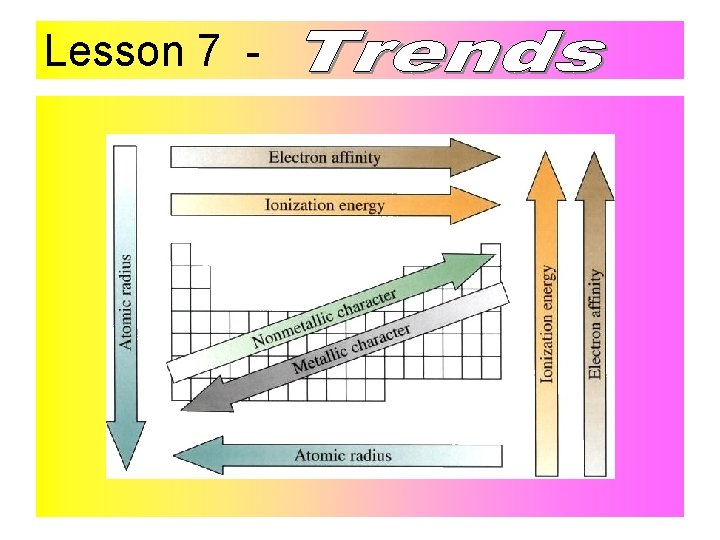
Lesson 7 Melting point and boiling point increase towards the middle and then decrease. Reactivity increases going down the group for metals and decreases going down the group for nonmetals. See this link from the Royal Society of Chemistry: http: //www. rsc. org/periodic-table/trends

Lesson 7 -
