Lesson 3 Substances Their Properties ESSE NTIAL QUESTIO

Lesson 3 Substances & Their Properties ESSE NTIAL QUESTIO N : HOW CAN YO U TELL ON E SUBSTANCE FROM ANO THER?
Introduction Everything around you is made up of atoms , and these atoms group together to form molecules and extended structures. While atoms, molecules, and extended structures are only visible on the atomic scale, when trillions of atoms and molecules come together that can be observed directly.

Observing Phenomena: Some liquids do not mix with other liquids, so they form distinct layers when they are poured into a bottle. What questions do you have about this phenomena?

1. Substances Substance: Matter that has a constant chemical composition and characteristics, generally composed of trillions and trillions of molecules ◦ Often called chemicals ◦ Scientists use the term substance to refer to a macroscopic amount of a molecule or extended structure. ◦ Macroscopic: On a scale people can see with their eyes; opposite of microscopic ◦ All substances are macroscopic amounts of atoms, molecules, or extended structures ◦ Most substances can be seen with your eyes, however, some are not, like oxygen

2. Properties Property: Characteristic of matter Examples: • Shape • Odor • Mass: The amount of matter in an object or sample • Measured in kilograms (kg) or grams (g) • Volume: The amount of space that an object occupies Some properties, like odor, are shared by all objects of that substance and never change, but others, such as shape, volume, or mass, are not shared by all objects of that substance and can change.
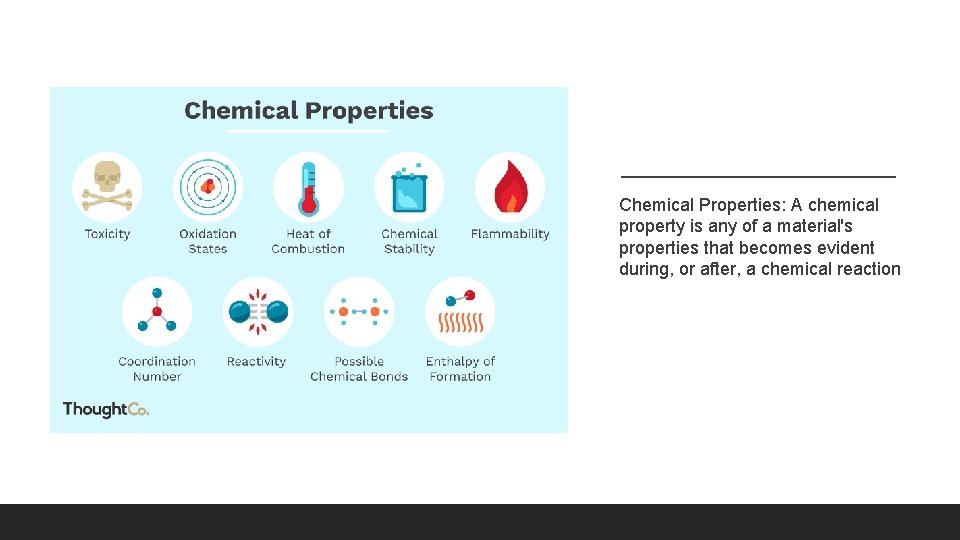
Chemical Properties: A chemical property is any of a material's properties that becomes evident during, or after, a chemical reaction

Physical Properties: can be observed or measured without changing the composition of matter. Physical properties are used to observe and describe matter. ◦ Physical appearance ◦ Texture ◦ Color ◦ Odor ◦ Melting & Boiling Point ◦ Density ◦ Solubility ◦ Polarity, and many others.


Matter that has a constant chemical composition and characteristics is know as a(n)___________

If something is ______, it is at a scale that can be seen with your eyes.

3. Density: a property that describes the mass of a certain volume of matter ◦ Density = Mass/Volume ◦ If 2 objects have the same volume but one has a higher density, then the one with the higher density has more mass that the other ◦ Density does not depend on the shape or size of the object

Which statement about density is true? A. Density is volume per unit mass B. Density does not depend on the size or shape of an object C. All objects of the same substance have different densities D. Density cannot be used to identify a substance

4. Melting & Boiling Point Scientists often use melting and boiling point to tell different substances apart ◦ Melting Point: The temperature at which a substance changes from a solid to a liquid as thermal energy is added to it ◦ Boiling Point: The temperature at which a substance changes from a liquid to a gas as thermal energy is added to it Melting or boiling point is a property that does not change on the amount of substance you have

5. Solubility: A property that describes how well one substance dissolves in another substance ◦ Liquids can be soluble in other liquids

6. Flammability: the ability of a chemical to burn or ignite, causing fire or combustion Scientists will often perform multiple tests on a substance to identify it because many substances have similar properties and cannot be identified by one test. They have to carefully perform each test and then gather the information from all of them. Then they compare all their results to narrow down what substance an object is made of

7. Mixtures Although everything in the world is made of atoms, not everything is a pure substance. Many objects you interact with are mixtures of substances. Mixture: A material made up of two or more different substances which are physically combined
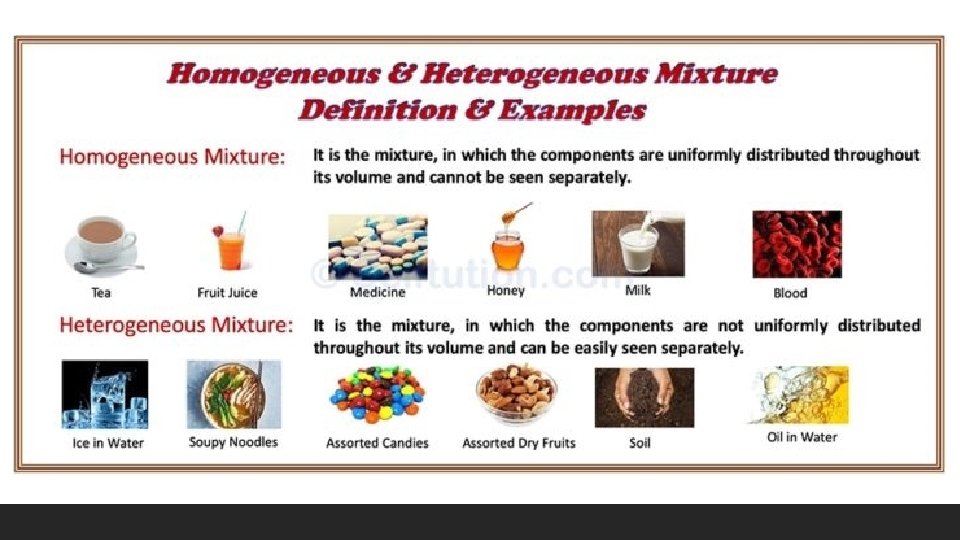


Which of the following statements is true? A. Everything in the world is not made of atoms. B. Air is a pure substance C. Many of the objects that you interact with are mixtures of substances D. Many of the objects that you interact with are pure substances

Lesson Summary Substances: When trillions of atoms or molecules come together, they form a substance. Usually, substances are macroscopic, or on a scale that you can see them. Properties: Scientists can describe objects using their properties, such as mass, shape, or odor. Some properties, such as odor, can be used to identify a substance an object is made of. These properties do not change based off of an object's size or shape. These properties only change if the substance the object is made of changes. Other properties, such as mass or shape, cannot be used to identify a substance. Density: Density is a property that is the amount of mass per unit volume. It can be used to identify a substance.

Lesson Summary Melting and Boiling Point: Melting and boiling point are properties that describe the temperature that a substance melts or boils. They can be used to identify a substance. Solubility: Solubility is a property that describes how well a substance dissolves in another substance. It can be used to identify a substance. Flammability: Flammability is a property that describes how well a substance burns. It can be used to identify a substance. Mixtures: Not everything is a substance; many of the objects you interact with are mixtures of different pure substances.
- Slides: 21