Lesson 3 LT I can predict how temperature


















































- Slides: 53

Lesson 3 LT: I can predict how temperature and pressure affect the volume of a gas.

Cool School Bus You have 3 minutes to answer the following questions.

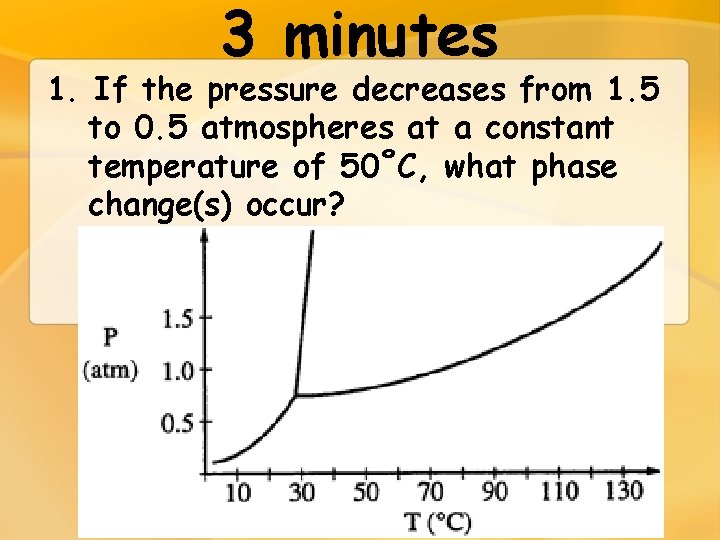
3 minutes 1. If the pressure decreases from 1. 5 to 0. 5 atmospheres at a constant temperature of 50˚C, what phase change(s) occur?

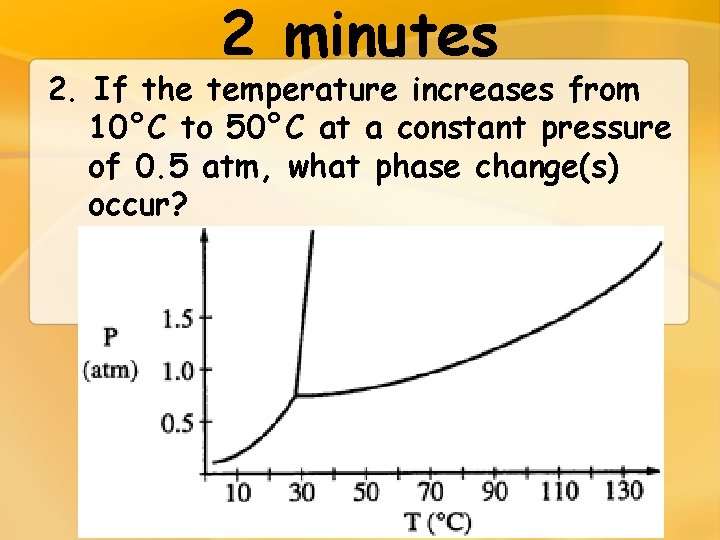
2 minutes 2. If the temperature increases from 10°C to 50°C at a constant pressure of 0. 5 atm, what phase change(s) occur?

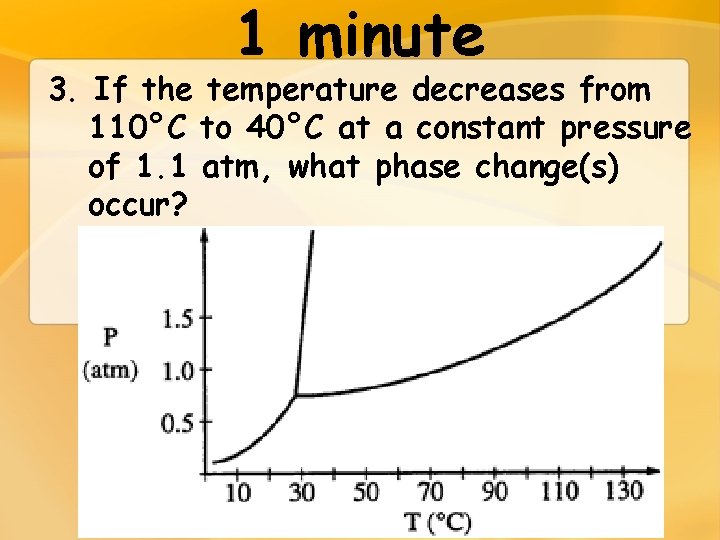
1 minute 3. If the temperature decreases from 110°C to 40°C at a constant pressure of 1. 1 atm, what phase change(s) occur?

5 seconds

4 seconds

3 seconds

2 seconds

1 second

Time for Class!

Lesson 3 Cool School Bus You have 3 minutes to answer the following question.

3 minutes At what temperature will 0. 654 moles of neon gas occupy 12. 30 liters at 1. 95 atmospheres?

2 minutes At what temperature will 0. 654 moles of neon gas occupy 12. 30 liters at 1. 95 atmospheres?

1 minute At what temperature will 0. 654 moles of neon gas occupy 12. 30 liters at 1. 95 atmospheres?

5 seconds

4 seconds

3 seconds

2 seconds

1 second

Time for Class!

Lesson 3 Cool School Bus You have 4 minutes to answer the following question.

4 minutes At a pressure of 750. 0 mm Hg and 25°C, 2. 00 L of He are heated until a volume of 20. 0 L and a pressure of 3. 50 atm are reached. What is the new temperature?

3 minutes At a pressure of 750. 0 mm Hg and 25°C, 2. 00 L of He are heated until a volume of 20. 0 L and a pressure of 3. 50 atm are reached. What is the new temperature?

2 minutes At a pressure of 750. 0 mm Hg and 25°C, 2. 00 L of He are heated until a volume of 20. 0 L and a pressure of 3. 50 atm are reached. What is the new temperature?

1 minute At a pressure of 750. 0 mm Hg and 25°C, 2. 00 L of He are heated until a volume of 20. 0 L and a pressure of 3. 50 atm are reached. What is the new temperature?

5 seconds

4 seconds

3 seconds

2 seconds

1 second

Time for Class!

Lesson 3 Cool School Bus You have 3 minutes to answer the following question.

3 minutes A gas takes up a volume of 17. 0 L, has a pressure of 2. 30 atm and a temp of 299 K. If I raise the temp to 350. K and lower the pressure to 1. 5 atm, What is the new volume?

2 minutes A gas takes up a volume of 17. 0 L, has a pressure of 2. 30 atm and a temp of 299 K. If I raise the temp to 350. K and lower the pressure to 1. 5 atm, What is the new volume?

1 minute A gas takes up a volume of 17. 0 L, has a pressure of 2. 30 atm and a temp of 299 K. If I raise the temp to 350. K and lower the pressure to 1. 5 atm, What is the new volume?

5 seconds

4 seconds

3 seconds

2 seconds

1 second

Time for Class!

Lesson 3 (honors) Cool School Bus You have 4 minutes to answer the following question.

4 minutes Ammonium sulfate, an important fertilizer, can be prepared according to the following balanced equation: 2 NH 3 (g) + H 2 SO 4 (NH 4)2 SO 4 (aq) Calculate the volume of NH 3 (in L) needed at 20. 0ºC and 25. 0 atm to react with 150. kg of H 2 SO 4.

3 minutes Ammonium sulfate, an important fertilizer, can be prepared according to the following balanced equation: 2 NH 3 (g) + H 2 SO 4 (NH 4)2 SO 4 (aq) Calculate the volume of NH 3 (in L) needed at 20. 0ºC and 25. 0 atm to react with 150. kg of H 2 SO 4.

2 minutes Ammonium sulfate, an important fertilizer, can be prepared according to the following balanced equation: 2 NH 3 (g) + H 2 SO 4 (NH 4)2 SO 4 (aq) Calculate the volume of NH 3 (in L) needed at 20. 0ºC and 25. 0 atm to react with 150. kg of H 2 SO 4.

1 minute Ammonium sulfate, an important fertilizer, can be prepared according to the following balanced equation: 2 NH 3 (g) + H 2 SO 4 (NH 4)2 SO 4 (aq) Calculate the volume of NH 3 (in L) needed at 20. 0ºC and 25. 0 atm to react with 150. kg of H 2 SO 4.

5 seconds

4 seconds

3 seconds

2 seconds

1 second

Time for Class!