Lesson 3 Chapter 1 Measurement A Common Language


















- Slides: 22

Lesson 3 Chapter 1 Measurement – A Common Language

Standard Measurement System �Metric system • Developed in France • Based on the number ten � Using the international system of units (SI) as the standard system of measurement allows scientists to compare data and communicate with each other about their results

What looks easier to remember? 2. 5 centimeter = 1 inch, 12 inches = 1 foot, 3 feet I yard, 1760 yards 1 mile OR 10 centimeter = 1 decimeter, 10 decimeter = 1 meter, 10 meter = 1 decameter, 10 decameter = 1 hectometer, 10 hectometer = 1 kilometer

Drive for 18 meters, turn left, then you will find the house 1 kilometer down on the right. American woman Fahre fȕr 18 meters, nach links biegen, dann wirst du denn House nach 1 kilometer am rechts finden. German

Prefixes �Milli- 0. 001 One thousandth of a unit �Centi- 0. 01 One hundredth of a unit �Deci- 0. 1 One tenth of a unit �None- 1 unit �Deka- 10 unit �Hecto- 100 unit �Kilo- 1000 unit

Figure 1 Complete figure 1 on page 23

Length �Distance from one point to another �SI for length is the meter

Mass �The measure of the amount of matter in an object �SI for mass is the Kilogram � 1000 milligram = 1 gram � 1000 grams = 1 kilogram Triplebeam balance

Weight �Is the measure of the force of gravity acting on an object. �A scale is used to measure weight

Volume �The amount of space an object or substance takes up. �The SI for measuring volume is cubic meter (m³), cubic centimeter (cm³), and liter (L). �Cubic meters and centimeters are used for measuring volume of a solid. �Liter is used for measuring liquids.

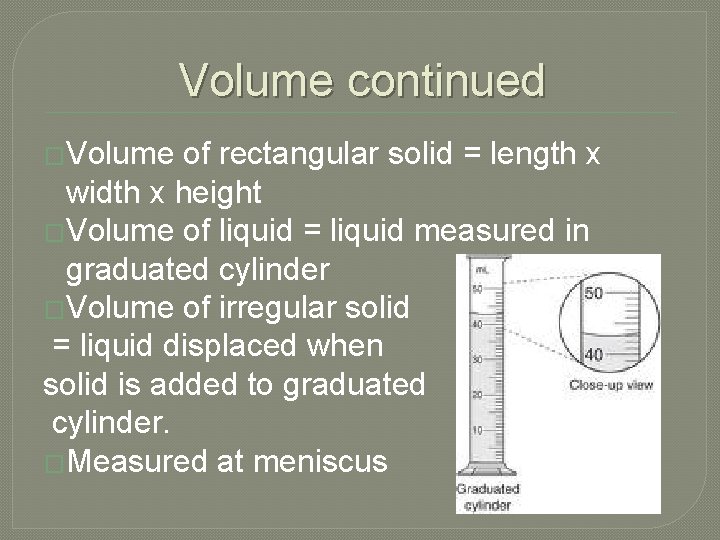
Volume continued �Volume of rectangular solid = length x width x height �Volume of liquid = liquid measured in graduated cylinder �Volume of irregular solid = liquid displaced when solid is added to graduated cylinder. �Measured at meniscus

Figure 4 Complete figure 4 on pages 26 and 27

Density �How much mass is contained in a given volume �The SI for measuring density if kilograms per cubic meter (kg/m³), grams per cubic centimeter (g/cm³), or grams per milliliter (g/ml).

Density continued �All densities of pure substances are the same despite how big or small �Water density is 1 g/cm³ so items with a density less than 1 g/cm³ will float on water, items more dense than 1 g/cm³ will sink. Both bars of gold will have the same density.

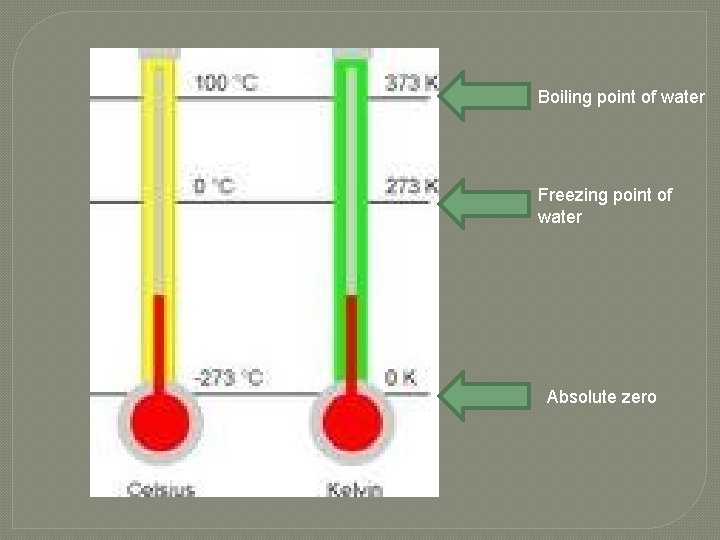
Temperature �Temperature is measured by scientists using both Celsius and Kelvin is the SI unit for temperature. �Kelvin has no negative numbers.

Boiling point of water Freezing point of water Absolute zero

Time �The second (s) is the SI unit used to measure time. �Also uses the prefixes.

Figure 7 Complete figure 7 on page 30

Conversions to know!

Video Take 5 notes on video

Homework STUDY OVER WEEKEND!!! We will have a 15 – 20 question quiz next week on the material learned from lesson 2 and 3!

Picture sources � topnews. in � ace-clipart. com � medicalscale 1. com � cutleryandmore. com � graphicsfactory. com � clipartguide. com � proprofs. com � solutionsguide. tetratec. com � patentmath. com � avogadro. co. uk � blurtit. com � italy. worldcupblog. org