Lesson 25 Molecular Mass and Moles Objectives The
Lesson 25 Molecular Mass and Moles Objectives: - - The student will determine the molecular mass of a formula. The student will convert between mass and moles for a formula.

Moles, Molecular formulas with calculations, and %composition. mpg
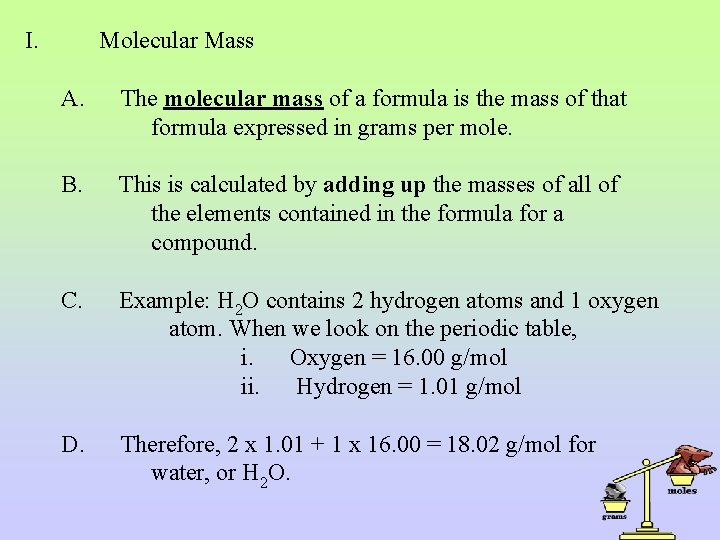
I. Molecular Mass A. The molecular mass of a formula is the mass of that formula expressed in grams per mole. B. This is calculated by adding up the masses of all of the elements contained in the formula for a compound. C. Example: H 2 O contains 2 hydrogen atoms and 1 oxygen atom. When we look on the periodic table, i. Oxygen = 16. 00 g/mol ii. Hydrogen = 1. 01 g/mol D. Therefore, 2 x 1. 01 + 1 x 16. 00 = 18. 02 g/mol for water, or H 2 O.
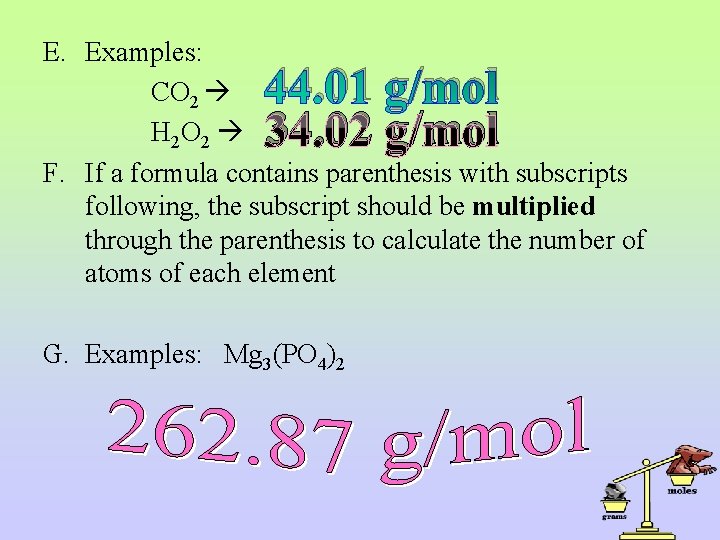
E. Examples: CO 2 H 2 O 2 F. If a formula contains parenthesis with subscripts following, the subscript should be multiplied through the parenthesis to calculate the number of atoms of each element 44. 01 g/mol 34. 02 g/mol G. Examples: Mg 3(PO 4)2
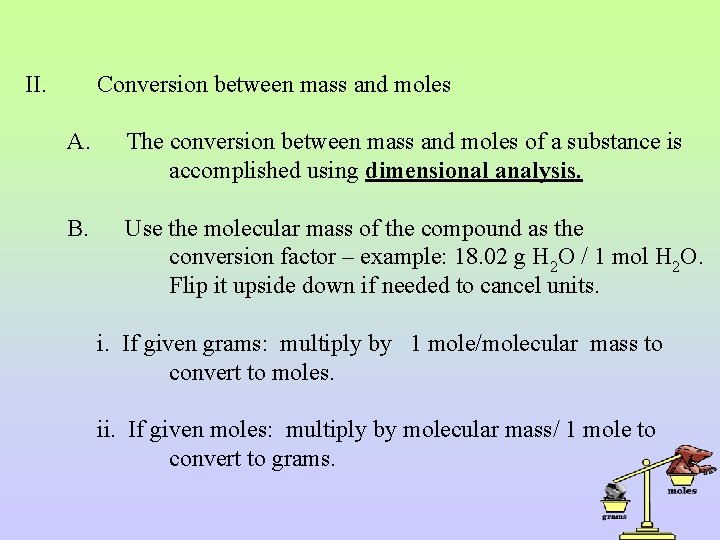
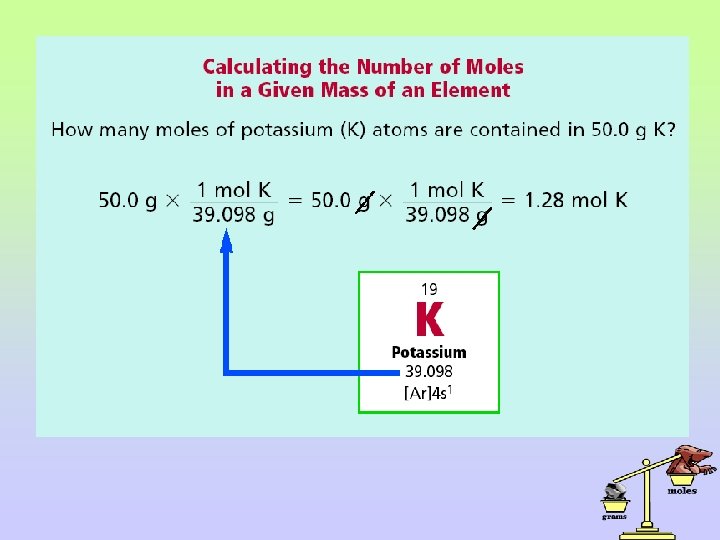
II. Conversion between mass and moles A. The conversion between mass and moles of a substance is accomplished using dimensional analysis. B. Use the molecular mass of the compound as the conversion factor – example: 18. 02 g H 2 O / 1 mol H 2 O. Flip it upside down if needed to cancel units. i. If given grams: multiply by 1 mole/molecular mass to convert to moles. ii. If given moles: multiply by molecular mass/ 1 mole to convert to grams.
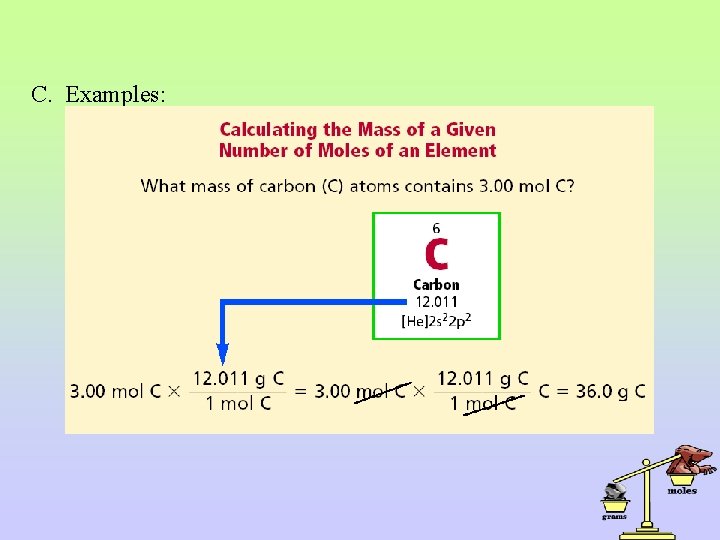
C. Examples:
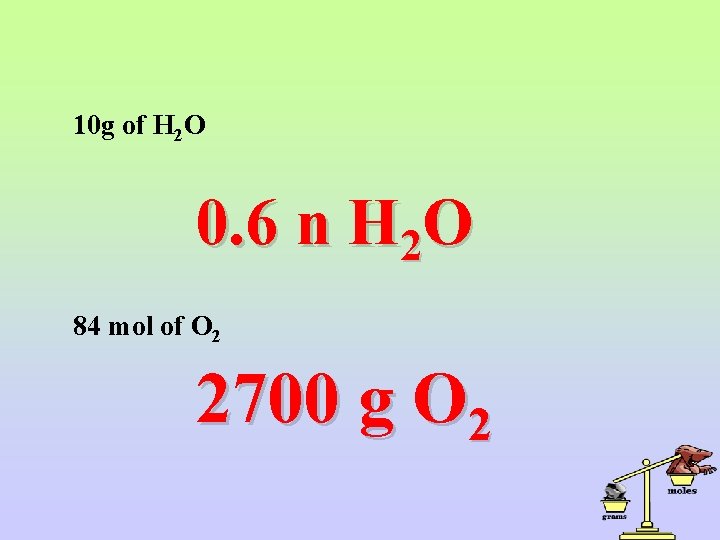
10 g of H 2 O 0. 6 n H 2 O 84 mol of O 2 2700 g O 2

III. Conversion between moles and particles A. B. The conversion between moles and number of particles (atoms, molecules, or formula units) is also accomplished with dimensional analysis. Avagadro’s Principle states that there are 6. 02 x 1023 particles in one mole. This then becomes a conversion factor with Avagadro’s number and 1 mole as the two terms. C. The direction in which you are converting determines which goes on top.
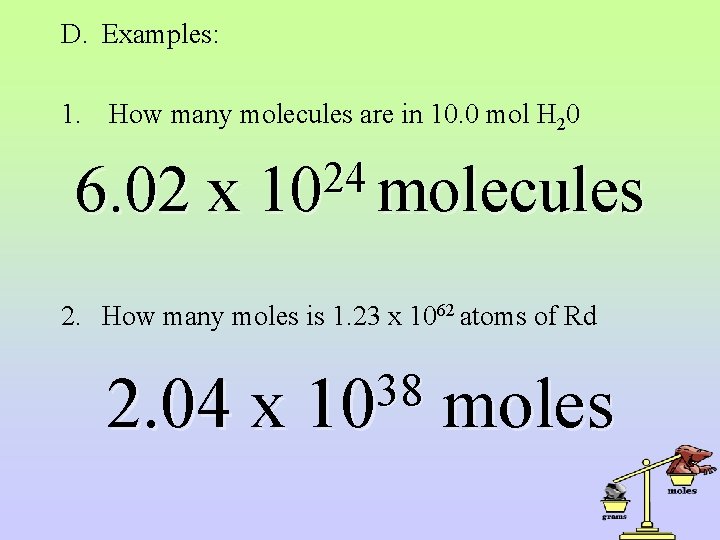
D. Examples: 1. How many molecules are in 10. 0 mol H 20 6. 02 x 24 10 molecules 2. How many moles is 1. 23 x 1062 atoms of Rd 2. 04 x 38 10 moles

IV. Conversion between mass and number of particles A. B. C. D. There is no direct conversion between mass and number of particles. This must be done through a two step process. First convert mass to moles as described in II above. Then convert moles to particles as described in III above.
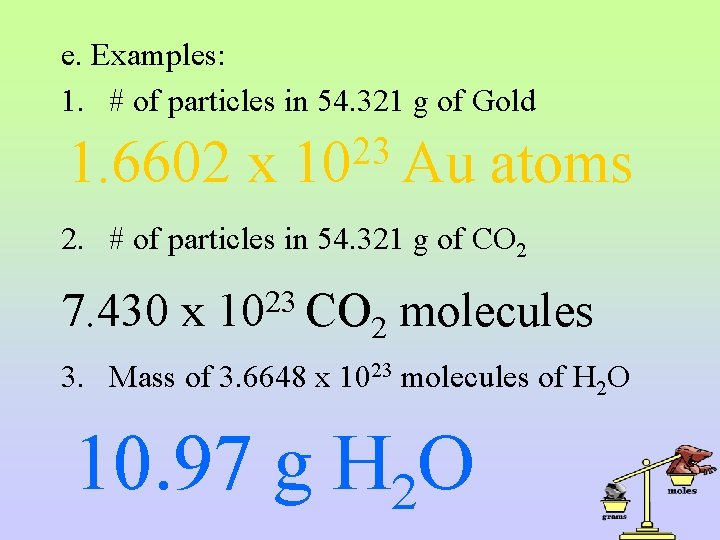
e. Examples: 1. # of particles in 54. 321 g of Gold 1. 6602 x 23 10 Au atoms 2. # of particles in 54. 321 g of CO 2 7. 430 x 23 10 CO 2 molecules 3. Mass of 3. 6648 x 1023 molecules of H 2 O 10. 97 g H 2 O


Formula Mass Problems Level 1 1. Find the molecular mass of each of the following compounds. a. KCl b. Na. F c. HI d. Li. Br e. Rb. Br f. Ba. Cl 2 g. Ca. I 2 h. Na 2 S i. Mg. S j. Al. P

2. Find the mass of each of the following, expressed in grams. a. 1. 00 mol of Ca. O b. 1. 00 mol of Be. Se c. 1. 00 mol of KF d. 1. 00 mol of Sr. O e. 2. 00 mol of Mg. I 2 f. 2. 00 mol of Li 3 P g. 5. 00 mol of Ca. Cl 2 h. 0. 50 mol of Fe. Br 2 i. 0. 20 mol of Cu 2 O j. 0. 40 mol of Hg 2 Cl 2

3. Find the amount of moles in each of the following masses. a. 18. 02 g of H 2 O b. 80. 92 g of HBr c. 17. 04 g of NH 3 d. 190. 42 g of Mg. Cl 2 e. 36. 75 g of K 2 S f. 25. 38 g of Sn. I 2 g. 11. 98 g of Fe. O h. 15. 0 g of KBr i. 25. 0 g of Sr. S j. 50. 0 g of Al. F 3

Level 2 1. Find the molecular mass of each of the following compounds. a. HNO 3 b. Fe 2 O 3 c. H 3 PO 4 d. K 2 SO 4 e. Be 5 As 2 f. NH 4 NO 3 g. Rb. SO 3 h. Li 2 CO 3 i. Mg(OH)2 j. Al 2(SO 4)3

2. Find the mass of each of the following, expressed in grams. a. 1. 00 mol of HC 2 H 3 O 2 b. 2. 50 mol of K 2 Cr. O 4 c. 0. 50 mol of Ca(Cl. O 3)2 d. 0. 25 mol of Ba(NO 3)2 e. 0. 375 mol of Na 2 Cr 2 O 7 f. 0. 25 mol of Na. C 2 H 3 O 2 g. 0. 152 mol of H 3 PO 4 h. 0. 0582 mol of Li 2 SO 4 i. 0. 418 mol of Fe(NO 3)3 j. 1. 872 mol of Cu(C 2 H 3 O 2)2

3. Find the amount of moles in each of the following masses. a. 100. 00 g of Ca. CO 3 b. 100. 00 g of Ni(NO 3)2 c. 50. 00 g of C 6 H 12 O 6 d. 25. 00 g of K 3 PO 4 e. 15. 57 g of Bi(OH)3 f. 3. 50 g of As. Cl 3 g. 0. 572 g of Ca 3 P 2 h. 1. 750 g of Ca(C 2 H 3 O 2)2 i. 4. 904 g of Al(NO 3)3 j. 27. 85 g of Fe 3(PO 4)2

4. Find the amount of formula units (particles) of each of the masses listed in question #3. a. 100. 00 g of Ca. CO 3 b. 100. 00 g of Ni(NO 3)2 c. 50. 00 g of C 6 H 12 O 6 d. 25. 00 g of K 3 PO 4 e. 15. 57 g of Bi(OH)3 f. 3. 50 g of As. Cl 3 g. 0. 572 g of Ca 3 P 2 h. 1. 750 g of Ca(C 2 H 3 O 2)2 i. 4. 904 g of Al(NO 3)3 j. 27. 85 g of Fe 3(PO 4)2


- Slides: 23