Lesson 12 2 and 12 4 Laws of











- Slides: 17

Lesson 12. 2 and 12. 4 Laws of Attraction Phase Change

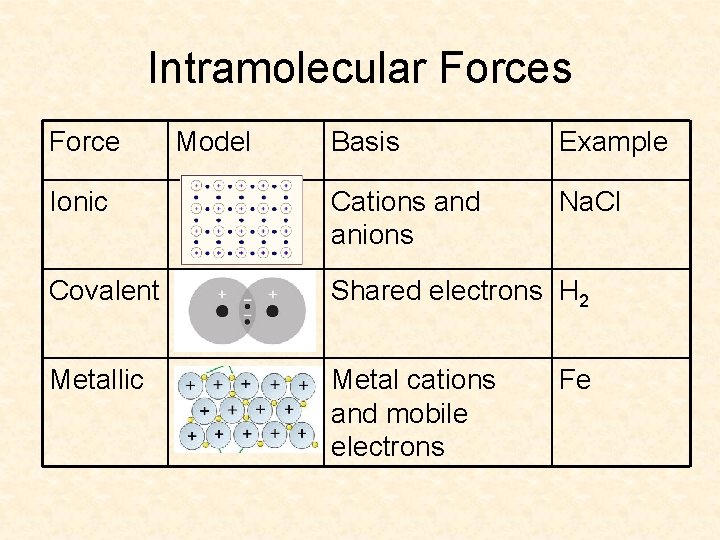
Intramolecular Forces Force Model Basis Example Ionic Cations and anions Na. Cl Covalent Shared electrons H 2 Metallic Metal cations and mobile electrons Fe

Dispersion Forces Weak Force Temporary shift in electron density clouds Explains State of Matter of Halogens Small to Large F 2 (g) Cl 2 (g) Br 2 (l) I 2 (s)

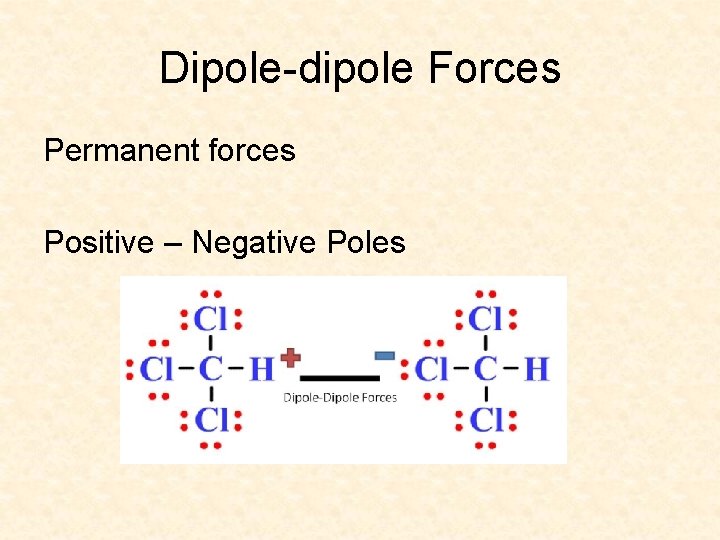
Dipole-dipole Forces Permanent forces Positive – Negative Poles

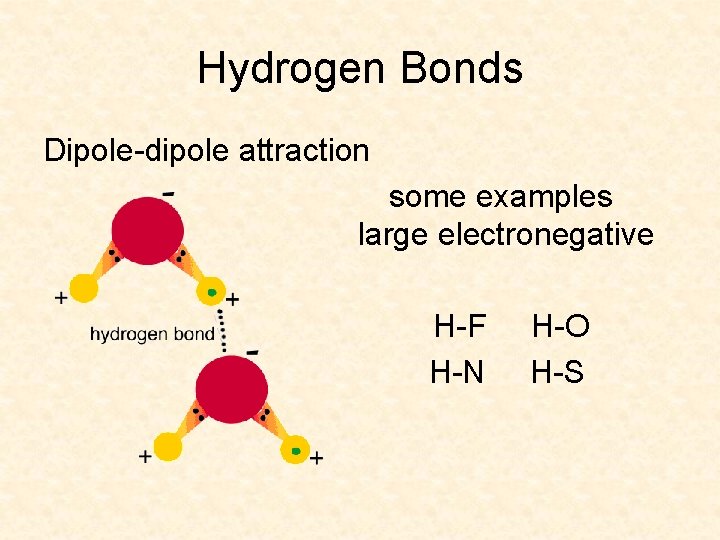
Hydrogen Bonds Dipole-dipole attraction some examples large electronegative H-F H-N H-O H-S

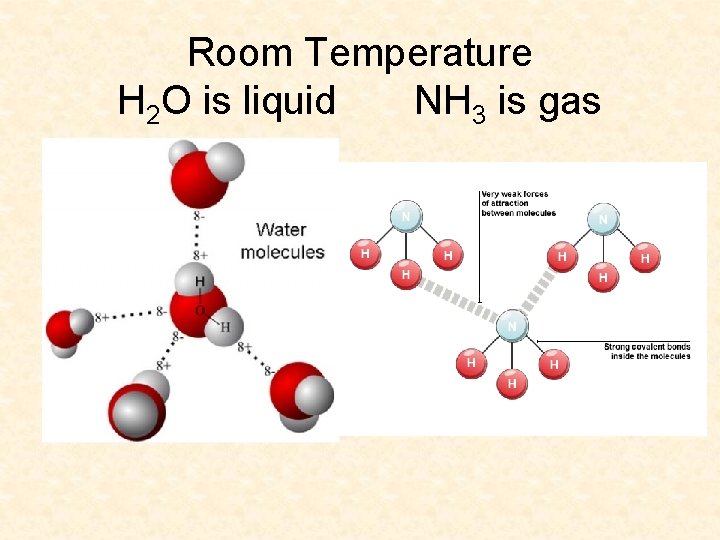
Room Temperature H 2 O is liquid NH 3 is gas

Questions Explain what determines a substance’s state at a given temperature. Compare intermolecular and intramolecular forces. Which molecules can form hydrogen bonds? a. H 2 b. H 2 S c. HCl d. HF

Questions Explain what determines a substance’s state at a given temperature. Intermolecular forces – solid very strong, liquid weaker, gas has none Compare intermolecular and intramolecular forces. Intramolecular hold particles together, intermolecular between particles Which molecules can form hydrogen bonds? a. H 2 b. H 2 S c. HCl d. HF

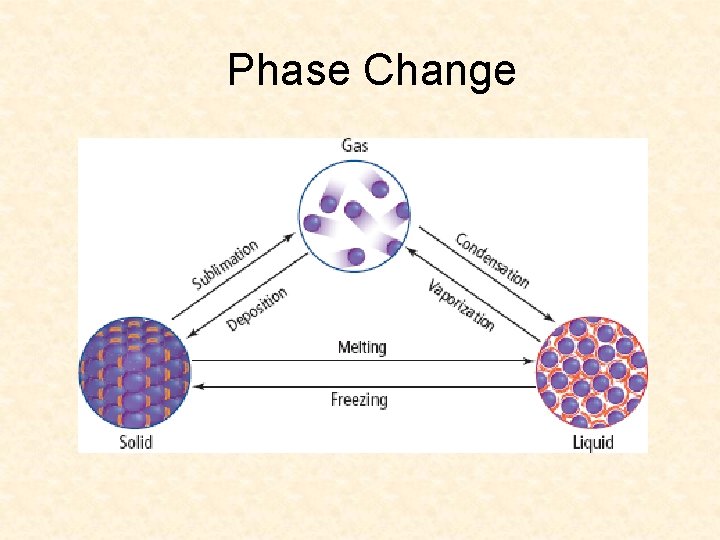
Phase Change

Phase Changes that Require Energy Melting – liquid is warmer than ice, heat is transferred Vaporization – liquid to vapor or gas gradually Sublimation – direct from solid to gas

Phase Change Release Energy Freezing – heat is removed Condensation – what happens to the glass of ice cold lemonade on a hot day?

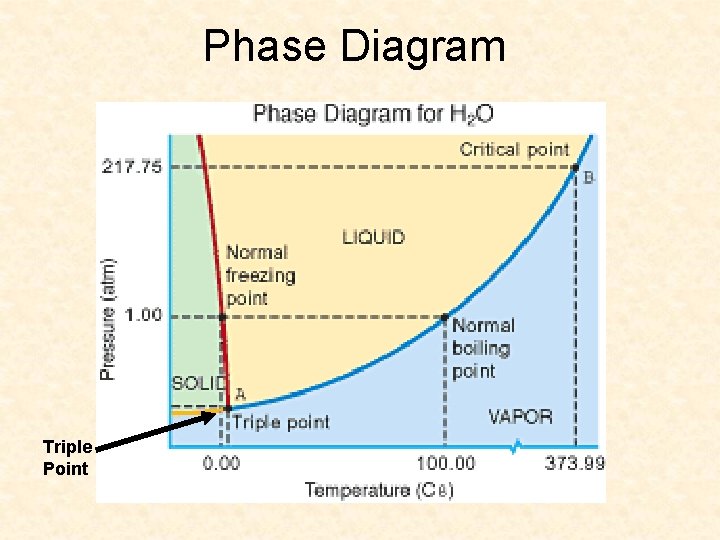
Phase Diagram Triple Point

Phase Diagram Questions Explain what the triple point and the critical point on a phase diagram supplies Determine the phase of water at 75. 00 C and 3. 00 atm.

Explain what the triple point and the critical point on a phase diagram supplies Triple point is where all 3 phases can coexist. Critical point is temp and pressure above which a substance is not a liquid Determine the phase of water at 75. 00 C and 3. 00 atm. liquid

Questions Explain how the addition or removal of energy can cause a phase change. Explain the difference between the processes of melting and freezing. Compare disposition and sublimation.

Questions Explain how the addition or removal of energy can cause a phase change. Add Energy, increase Kinetic Energy, less intermolecular forces Remove Energy, decrease Kinetic Energy, increase intermolecular forces

Explain the difference between the processes of melting and freezing. Freezing: Liquid to solid, release Energy Melting: Solid to liquid, need to input Energy Compare disposition and sublimation. Sublimation: solid to vapor directly, skips liquid phase Disposition: Vapor to solid, without liquid phase