Lesson 1 Light and Quantized Energy Build to

Lesson 1 Light and Quantized Energy

Build to the Essential Question How do the wave and particle natures of light compare? What is a quantum of energy and how is it related to an energy change of matter? How do continuous electromagnetic spectra and atomic emission spectra compare and contrast?

New Vocabulary electromagnetic radiation quantum wavelength Planck’s constant frequency photoelectric effect amplitude photon electromagnetic spectrum atomic emission spectrum

Review Vocabulary radiation: the rays and particles— alpha particles, beta particles, and gamma rays—that are emitted by radioactive material
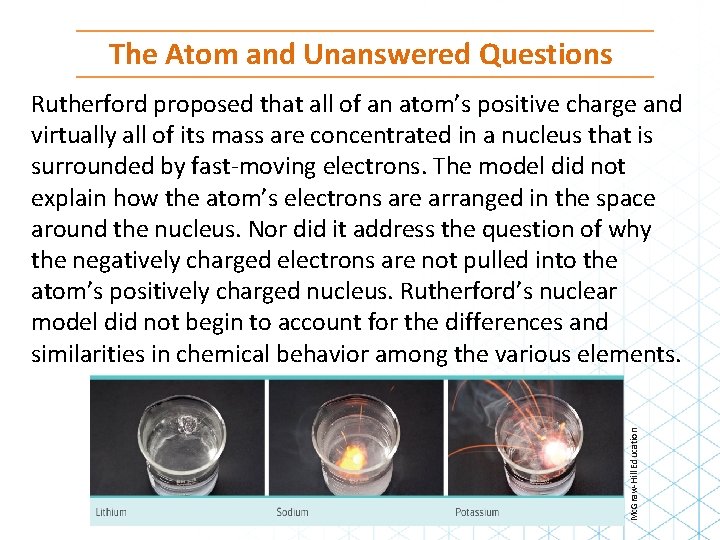
The Atom and Unanswered Questions Mc. Graw-Hill Education Rutherford proposed that all of an atom’s positive charge and virtually all of its mass are concentrated in a nucleus that is surrounded by fast-moving electrons. The model did not explain how the atom’s electrons are arranged in the space around the nucleus. Nor did it address the question of why the negatively charged electrons are not pulled into the atom’s positively charged nucleus. Rutherford’s nuclear model did not begin to account for the differences and similarities in chemical behavior among the various elements.
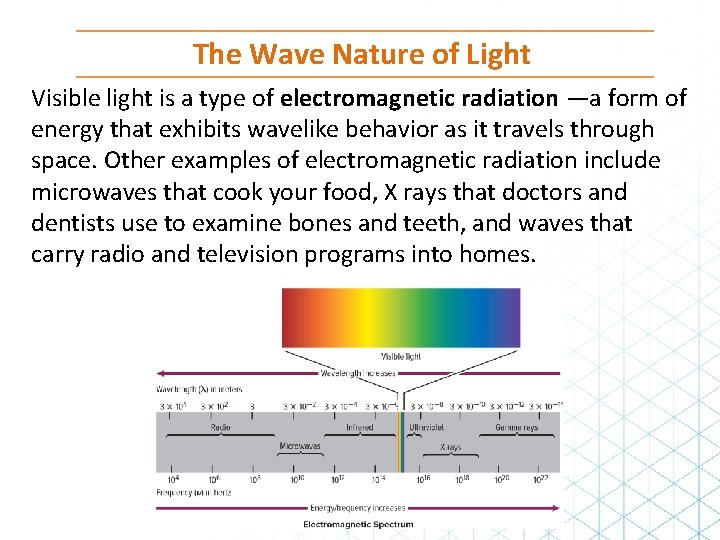
The Wave Nature of Light Visible light is a type of electromagnetic radiation —a form of energy that exhibits wavelike behavior as it travels through space. Other examples of electromagnetic radiation include microwaves that cook your food, X rays that doctors and dentists use to examine bones and teeth, and waves that carry radio and television programs into homes.
The Particle Nature of Light ©Jack Sullivan/Alamy While considering light as a wave explains much of its everyday behavior, it fails to adequately describe important aspects of light’s interactions with matter. The wave model of light cannot explain why heated objects emit only certain frequencies of light at a given temperature, or why some metals emit electrons when light of a specific frequency shines on them. Scientists realized that a new model or a revision of the wave model of light was needed to address these phenomena.
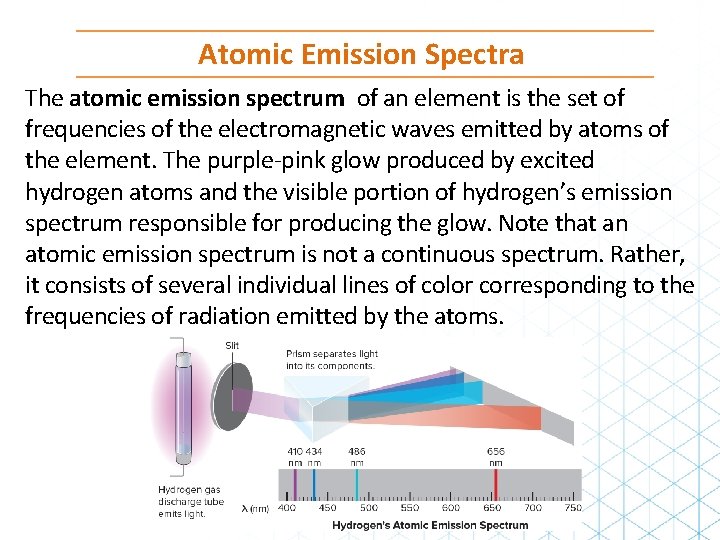
Atomic Emission Spectra The atomic emission spectrum of an element is the set of frequencies of the electromagnetic waves emitted by atoms of the element. The purple-pink glow produced by excited hydrogen atoms and the visible portion of hydrogen’s emission spectrum responsible for producing the glow. Note that an atomic emission spectrum is not a continuous spectrum. Rather, it consists of several individual lines of color corresponding to the frequencies of radiation emitted by the atoms.
- Slides: 8