Lectures on respiratory physiology Blood gas transport CONCENTRATION
Lectures on respiratory physiology Blood gas transport
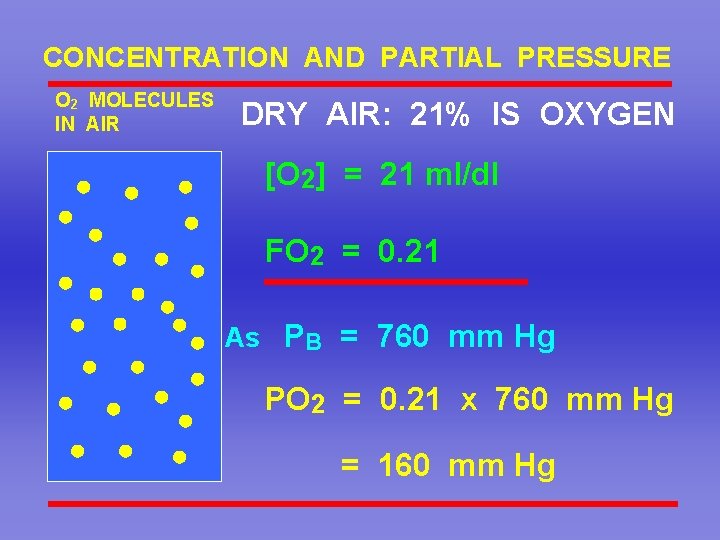
CONCENTRATION AND PARTIAL PRESSURE O 2 MOLECULES IN AIR DRY AIR: 21% IS OXYGEN [O 2] = 21 ml/dl FO 2 = 0. 21 As PB = 760 mm Hg PO 2 = 0. 21 x 760 mm Hg = 160 mm Hg
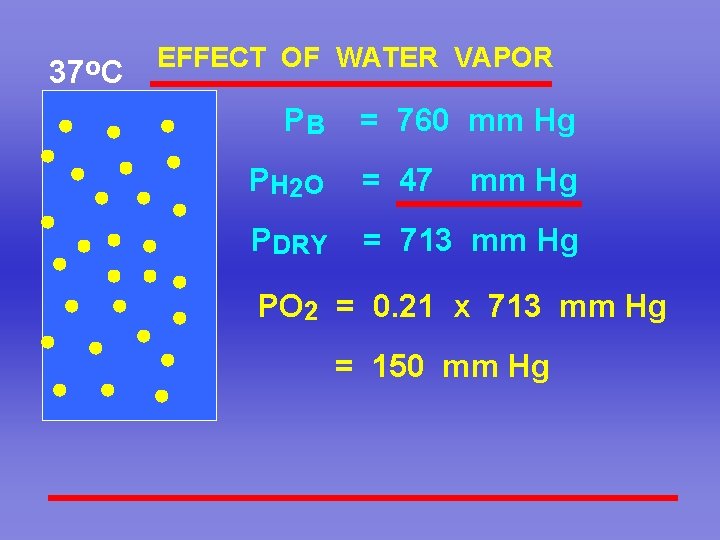
37 o. C EFFECT OF WATER VAPOR PB = 760 mm Hg P H 2 O = 47 mm Hg PDRY = 713 mm Hg PO 2 = 0. 21 x 713 mm Hg = 150 mm Hg
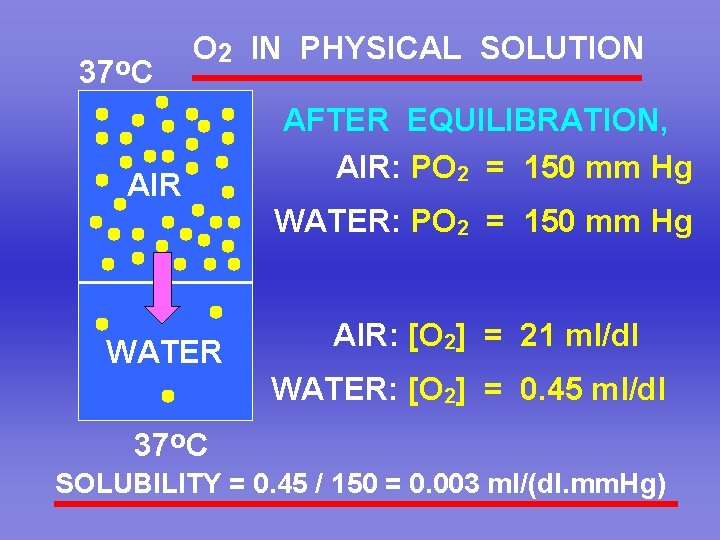
37 o. C O 2 IN PHYSICAL SOLUTION AIR AFTER EQUILIBRATION, AIR: PO 2 = 150 mm Hg WATER AIR: [O 2] = 21 ml/dl WATER: [O 2] = 0. 45 ml/dl 37 o. C SOLUBILITY = 0. 45 / 150 = 0. 003 ml/(dl. mm. Hg)

TRANSPORT OF O 2 IN SOLUTION DURING EXERCISE Solubility = 0. 003 ml/(dl. mm. Hg) PO 2 in arterial blood = 100 mm Hg [O 2] = 0. 3 ml/dl = 3 ml/liter Cardiac output = 30 liters/min Maximum O 2 available = 90 ml/min But O 2 requirement is 3000 ml/min
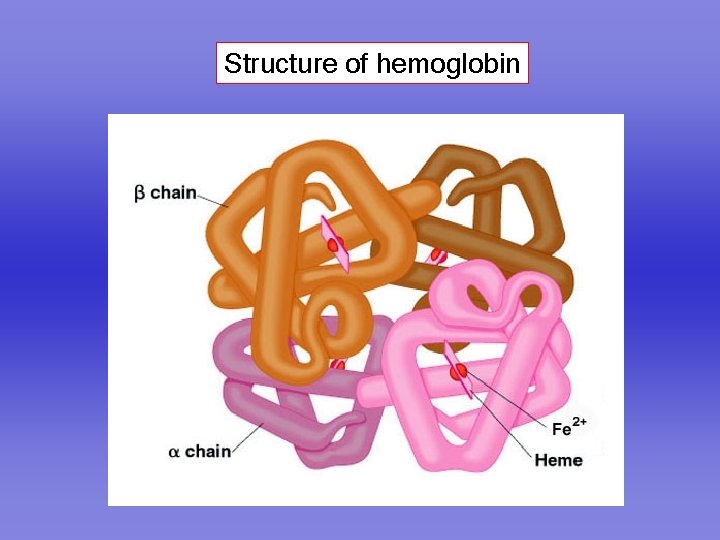
Structure of hemoglobin
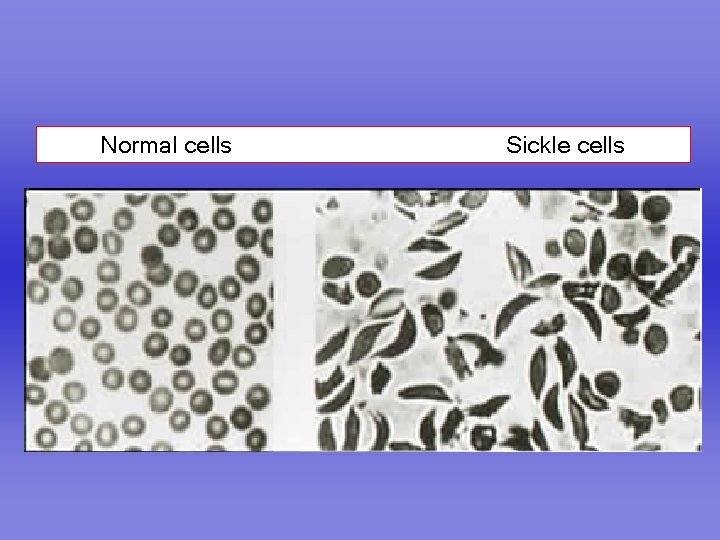
Normal cells Sickle cells
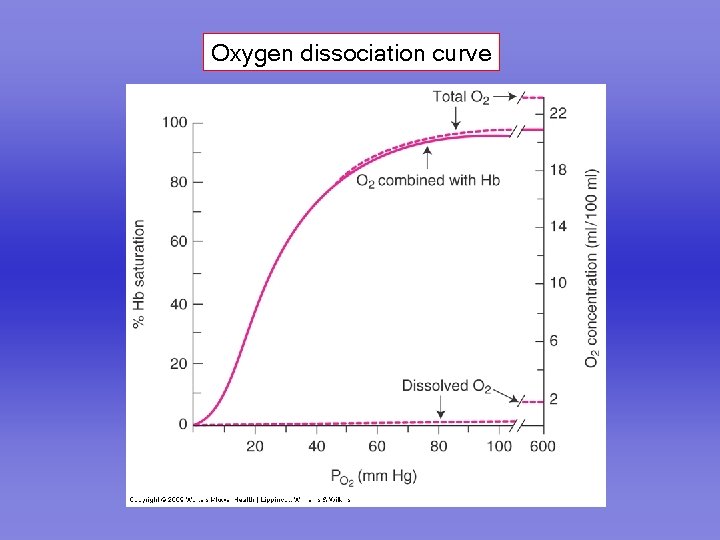
Oxygen dissociation curve
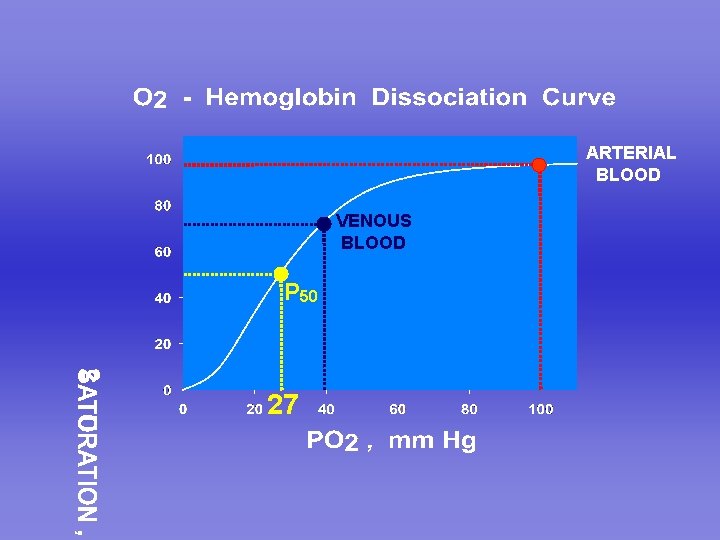
ARTERIAL BLOOD VENOUS BLOOD P 50 27
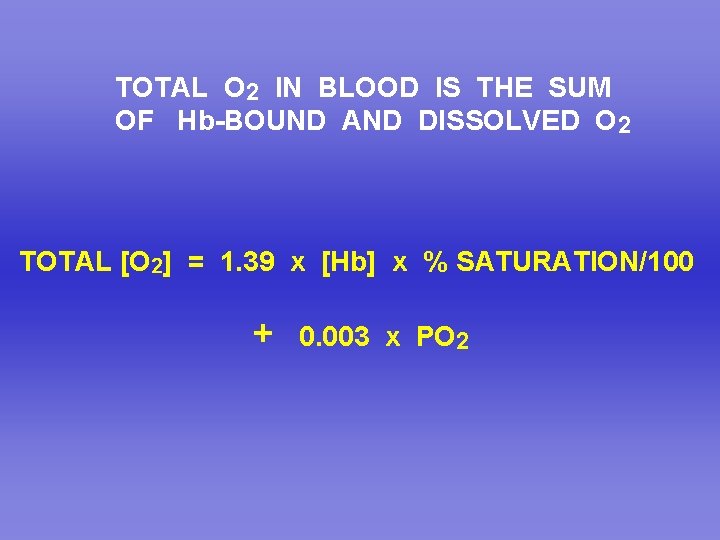
TOTAL O 2 IN BLOOD IS THE SUM OF Hb-BOUND AND DISSOLVED O 2 TOTAL [O 2] = 1. 39 x [Hb] x % SATURATION/100 + 0. 003 x PO 2
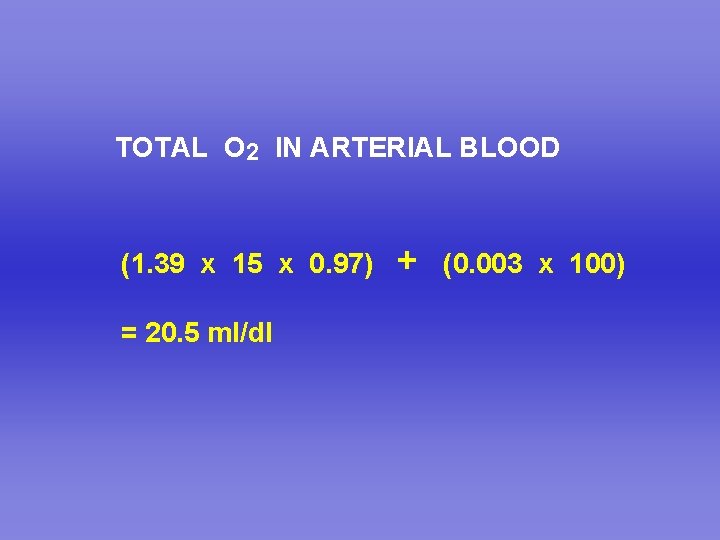
TOTAL O 2 IN ARTERIAL BLOOD (1. 39 x 15 x 0. 97) = 20. 5 ml/dl + (0. 003 x 100)
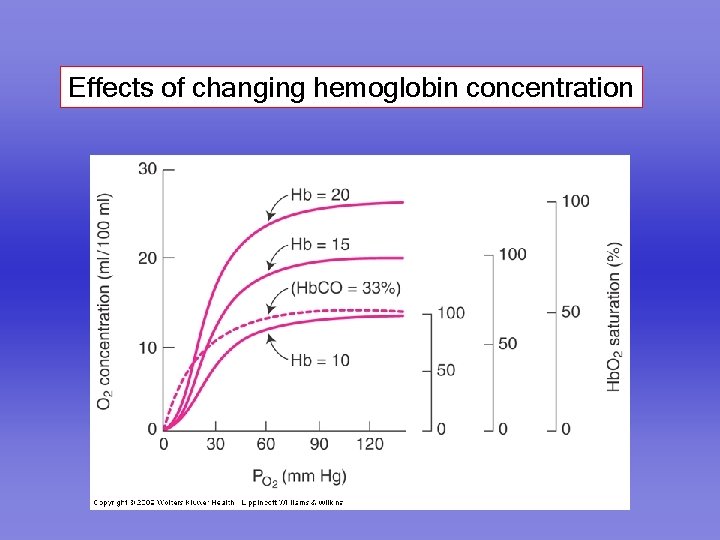
Effects of changing hemoglobin concentration
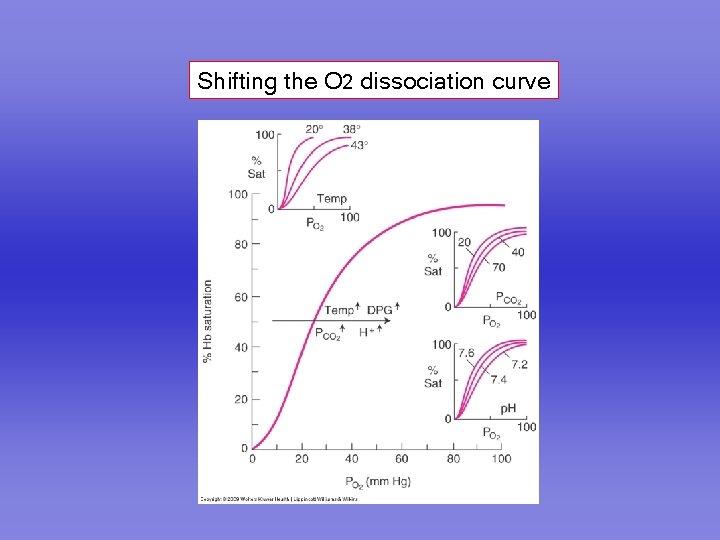
Shifting the O 2 dissociation curve
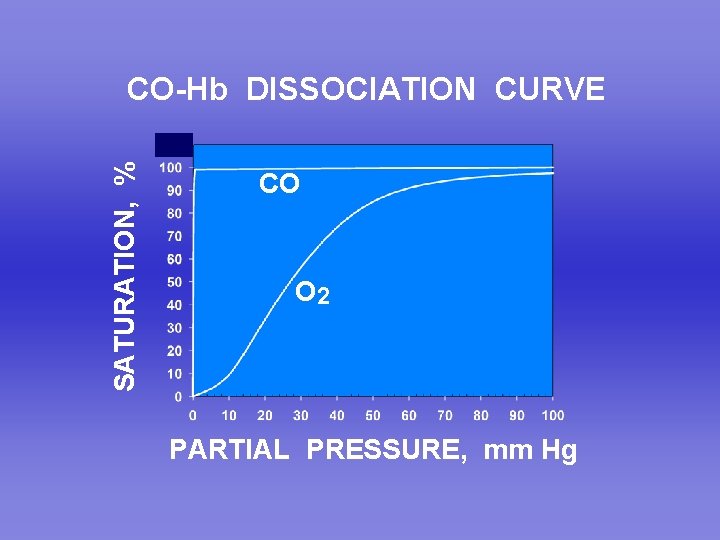
SATURATION, % CO-Hb DISSOCIATION CURVE CO O 2 PARTIAL PRESSURE, mm Hg
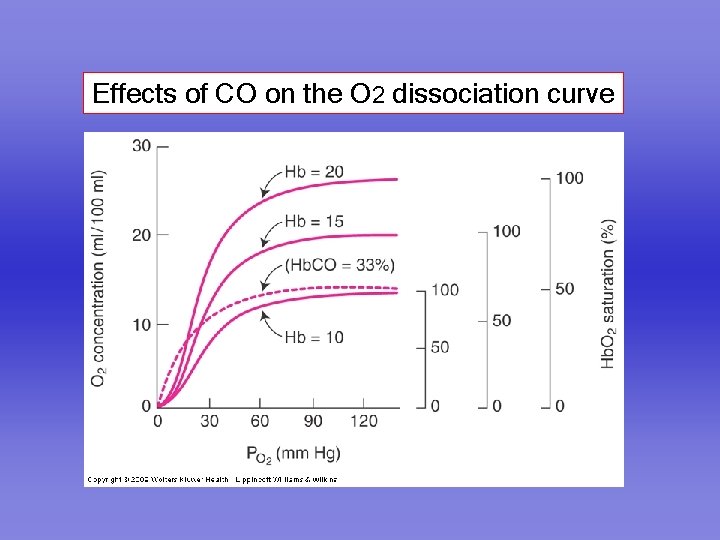
Effects of CO on the O 2 dissociation curve

Carbon dioxide is carried in the blood in three forms 1. Dissolved 2. As bicarbonate 3. As carbamino compounds

Solubility of Oxygen 0. 003 ml/(dl. mm. Hg) Solubility of Carbon Dioxide 0. 067 ml/(dl. mm. Hg)
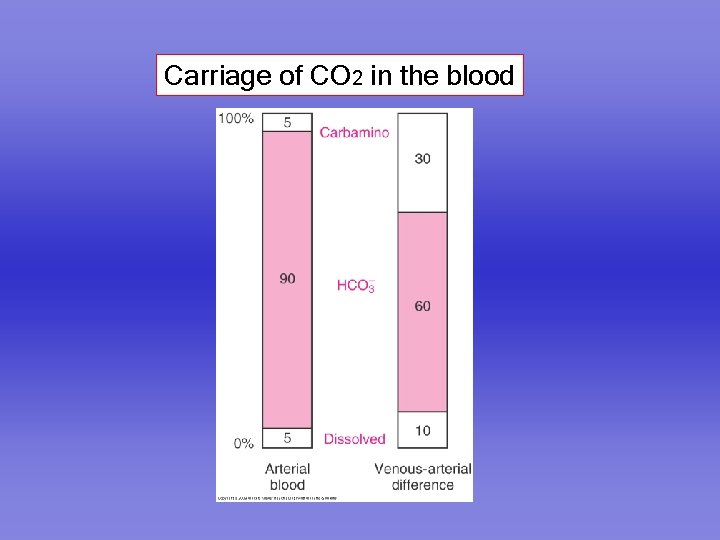
Carriage of CO 2 in the blood
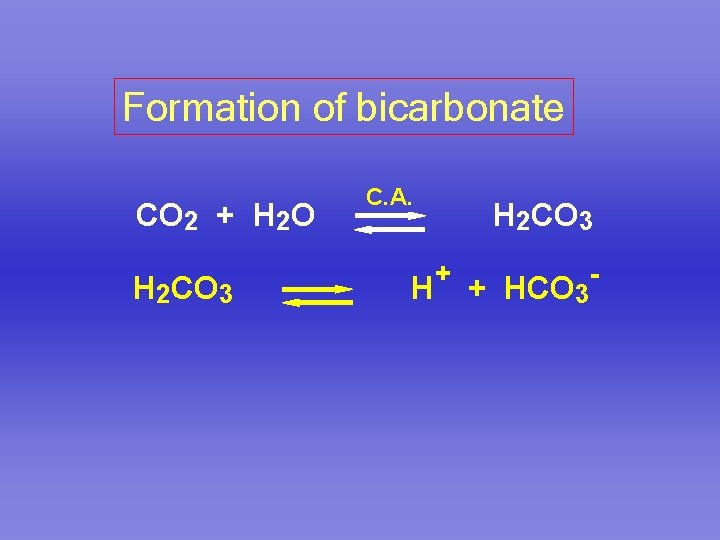
Formation of bicarbonate CO 2 + H 2 O H 2 CO 3 C. A. H H 2 CO 3 + + HCO 3 -
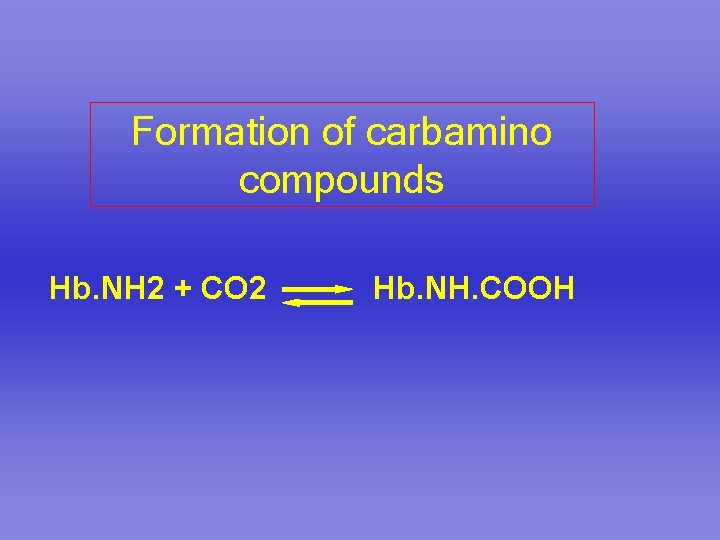
Formation of carbamino compounds Hb. NH 2 + CO 2 Hb. NH. COOH
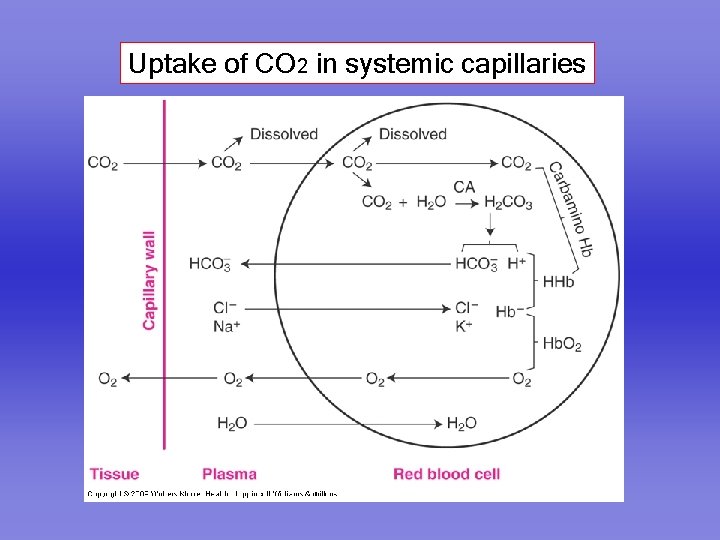
Uptake of CO 2 in systemic capillaries
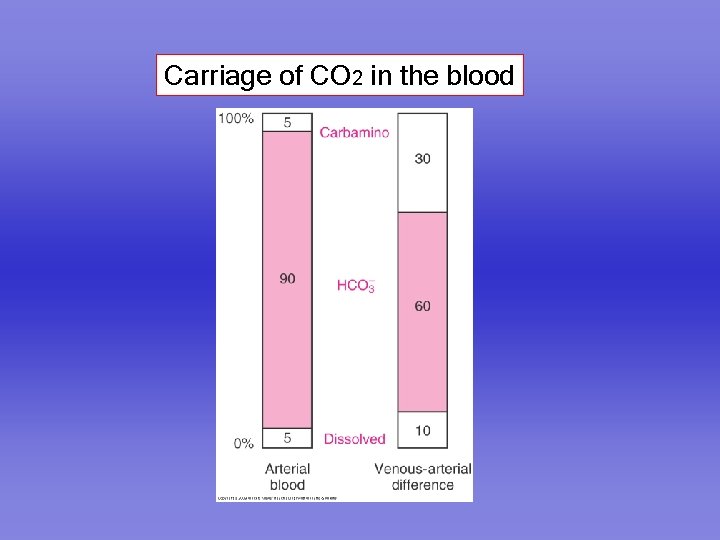
Carriage of CO 2 in the blood
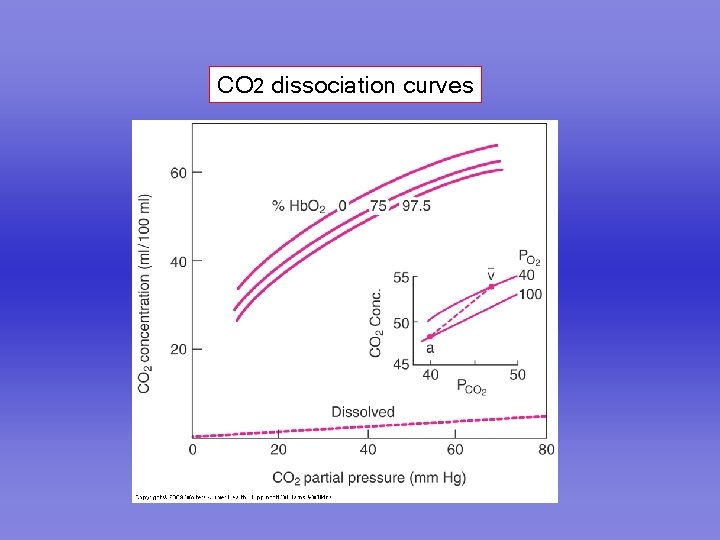
CO 2 dissociation curves
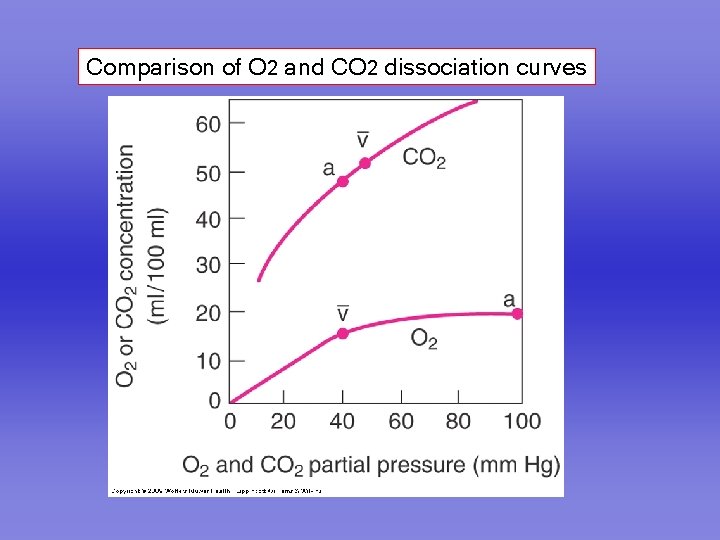
Comparison of O 2 and CO 2 dissociation curves
- Slides: 24