Lectures 3 4 Oneelectron atoms o Schrdinger equation







- Slides: 26

Lectures 3 -4: One-electron atoms o Schrödinger equation for one-electron atom. o Solving the Schrödinger equation. o Wavefunctions and eigenvalues. o Atomic orbitals. o See Chapter 7 of Eisberg & Resnick. PY 3 P 05

The Schrödinger equation o One-electron atom is simplest bound system in nature. o Consists of positive and negative particles moving in 3 D Coulomb potential: o Z =1 for atomic hydrogen, Z =2 for ionized helium, etc. o Electron in orbit about proton treated using reduced mass: o Total energy of system is therefore, PY 3 P 05

The Schrödinger equation o Using the Equivalence Principle, the classical dynamical quantities can be replaced with their associated differential operators: o Substituting, we obtain the operator equation: o Assuming electron can be described by a wavefunction of form, can write or where, is the Laplacian operator. PY 3 P 05

The Schrödinger equation o Since V(x, y, z) does not depend on time, Schrödinger equation and the eigenfunction Schrödinger equation: o As V = V(r), convenient to use spherical polar coordinates. is a solution to the is a solution of the time-independent (1) where o Can now use separation of variables to split the partial differential equation into a set of ordinary differential equations. PY 3 P 05

Separation of the Schrödinger equation (2) o Assuming the eigenfunction is separable: o Using the Laplacian, and substituting (2) and (1): o Carrying out the differentiations, o Note total derivatives now used, as R is a function of r alone, etc. o Now multiply through by and taking transpose, (3) PY 3 P 05

Separation of the Schrödinger equation o As the LHS of Eqn 3 does nor depend on r or and RHS does not depend on their common value cannot depend on any of these variables. o Setting the LHS of Eqn 3 to a constant: (4) and RHS becomes o Both sides must equal a constant, which we choose as l(l+1): (5) (6) o We have now separated the time-independent Schrödinger equation into three ordinary differential equations, which each only depend on one of (4), (5) and R(6). . PY 3 P 05

Summary of separation of Schrödinger equation o Express electron wavefunction as product of three functions: o As V ≠ V(t), attempt to solve time-independent Schrodinger equation. o Separate into three ordinary differential equations for and . o Eqn. 4 for ( ) only has acceptable solutions for certain value of ml. o Using these values for ml in Eqn. 5, ( ) only has acceptable values for certain values of l. o With these values for l in Eqn. 6, R(r) only has acceptable solutions for certain values of En. o Schrödinger equation produces three quantum numbers! PY 3 P 05

Azimuthal solutions ( ( )) o A particular solution of (4) is o As the einegfunctions must be single valued, i. e. , => and using Euler’s formula, o This is only satisfied if ml = 0, ± 1, ± 2, . . . o Therefore, acceptable solutions to (4) only exist when ml can only have certain integer values, i. e. it is a quantum number. o ml is called the magnetic quantum number in spectroscopy. o Called magnetic quantum number because plays role when atom interacts with magnetic fields. PY 3 P 05

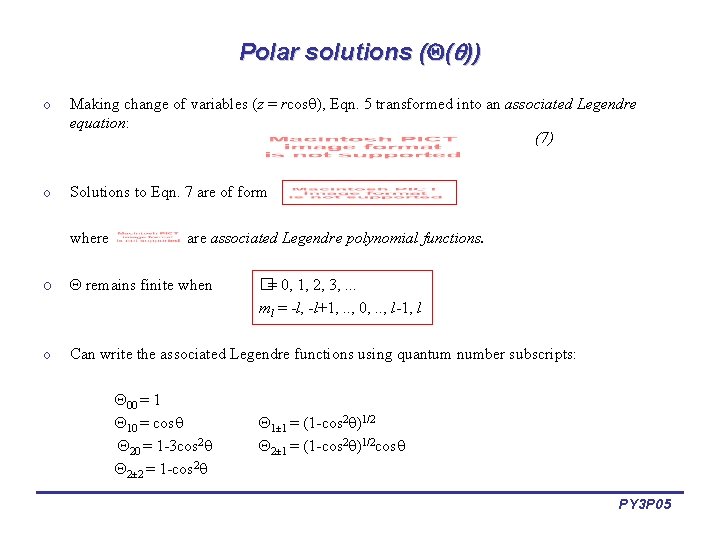
Polar solutions ( ( )) o Making change of variables (z = rcos , Eqn. 5 transformed into an associated Legendre equation: (7) o Solutions to Eqn. 7 are of form where associated Legendre polynomial functions. o remains finite when o Can write the associated Legendre functions using quantum number subscripts: 00 = 1 10 = cos 20 = 1 -3 cos 2 2± 2 = 1 -cos 2 �= 0, 1, 2, 3, . . . ml = -l, -l+1, . . , 0, . . , l-1, l 1± 1 = (1 -cos 2 )1/2 2± 1 = (1 -cos 2 )1/2 cos PY 3 P 05

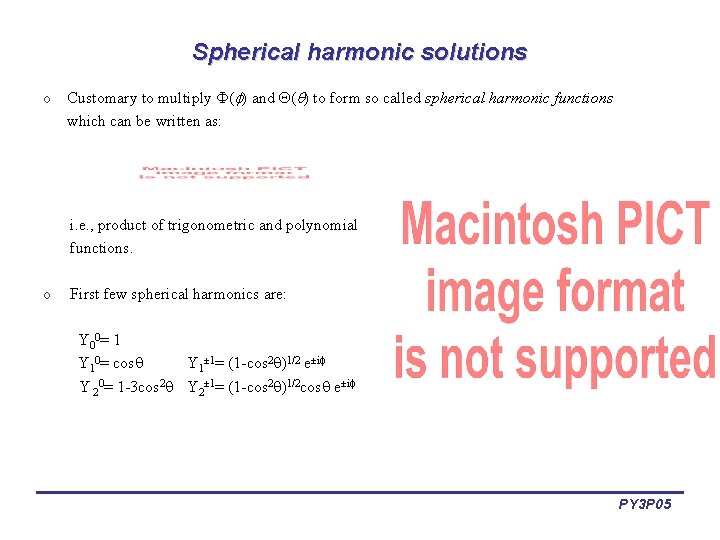
Spherical harmonic solutions o Customary to multiply ( ) and ( ) to form so called spherical harmonic functions which can be written as: i. e. , product of trigonometric and polynomial functions. o First few spherical harmonics are: Y 00= 1 Y 10= cos Y 1± 1= (1 -cos 2 )1/2 e±i Y 20= 1 -3 cos 2 Y 2± 1= (1 -cos 2 )1/2 cos e±i PY 3 P 05

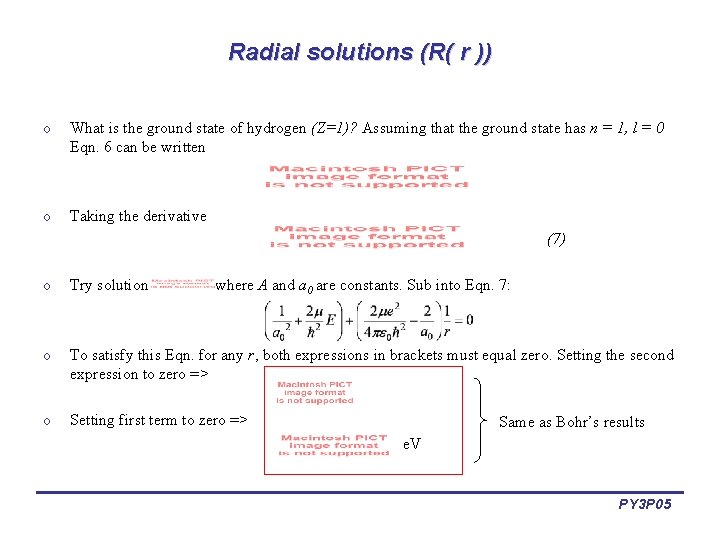
Radial solutions (R( r )) o What is the ground state of hydrogen (Z=1)? Assuming that the ground state has n = 1, l = 0 Eqn. 6 can be written o Taking the derivative (7) o Try solution o To satisfy this Eqn. for any r, both expressions in brackets must equal zero. Setting the second expression to zero => o Setting first term to zero => , where A and a 0 are constants. Sub into Eqn. 7: Same as Bohr’s results e. V PY 3 P 05

Radial solutions (R( r )) o Radial wave equation has many solutions, one for each positive integer of n. o Solutions are of the form (see Appendix N of Eisberg & Resnick): where a 0 is the Bohr radius. Bound-state solutions are only acceptable if e. V where n is the principal quantum number, defined by n = l +1, l +2, l +3, … o En only depends on n: all l states for a given n are degenerate (i. e. have the same energy). PY 3 P 05

Radial solutions (R( r )) o Gnl(Zr/a 0) are called associated Laguerre polynomials, which depend on n and l. o Several resultant radial wavefunctions (Rnl( r )) for the hydrogen atom are given below PY 3 P 05

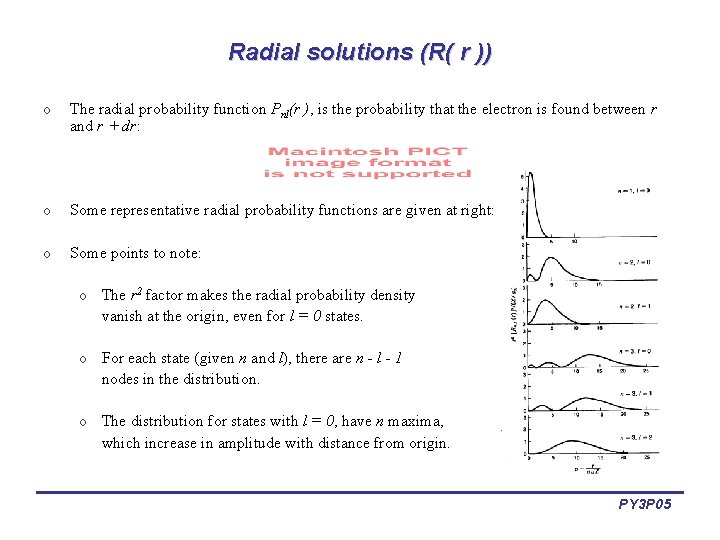
Radial solutions (R( r )) o The radial probability function Pnl(r ), is the probability that the electron is found between r and r + dr: o Some representative radial probability functions are given at right: o Some points to note: o The r 2 factor makes the radial probability density vanish at the origin, even for l = 0 states. o For each state (given n and l), there are n - l - 1 nodes in the distribution. o The distribution for states with l = 0, have n maxima, which increase in amplitude with distance from origin. PY 3 P 05

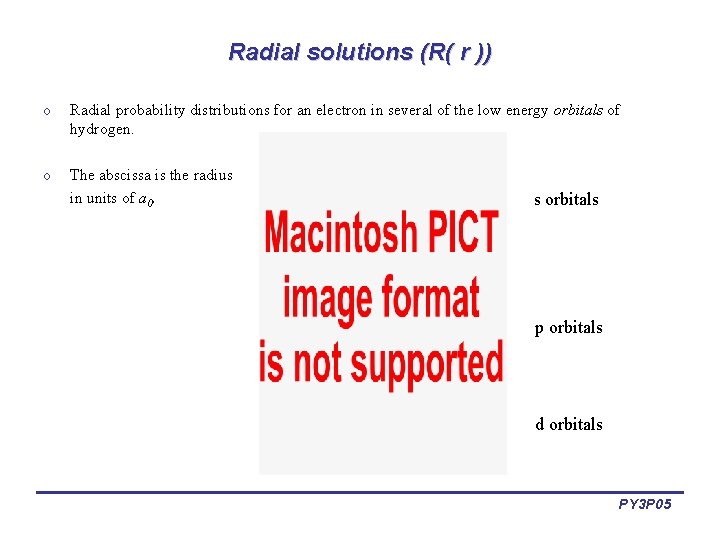
Radial solutions (R( r )) o Radial probability distributions for an electron in several of the low energy orbitals of hydrogen. o The abscissa is the radius in units of a 0. s orbitals p orbitals d orbitals PY 3 P 05

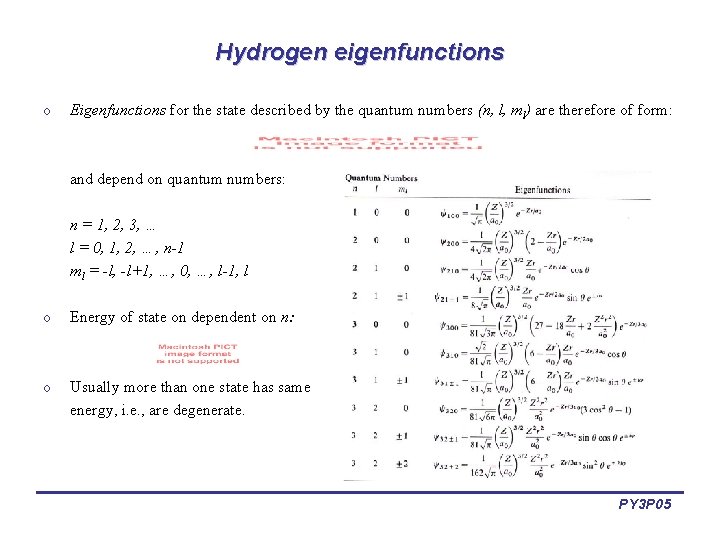
Hydrogen eigenfunctions o Eigenfunctions for the state described by the quantum numbers (n, l, ml) are therefore of form: and depend on quantum numbers: n = 1, 2, 3, … l = 0, 1, 2, …, n-1 ml = -l, -l+1, …, 0, …, l-1, l o Energy of state on dependent on n: o Usually more than one state has same energy, i. e. , are degenerate. PY 3 P 05

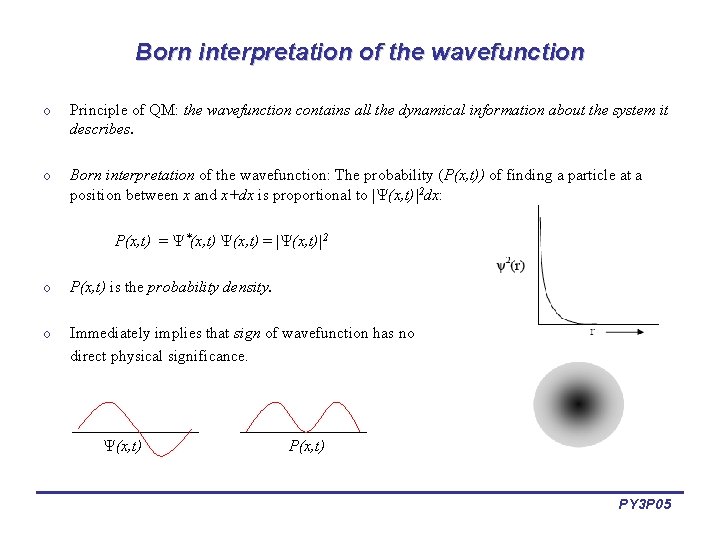
Born interpretation of the wavefunction o Principle of QM: the wavefunction contains all the dynamical information about the system it describes. o Born interpretation of the wavefunction: The probability (P(x, t)) of finding a particle at a position between x and x+dx is proportional to | (x, t)|2 dx: P(x, t) = *(x, t) = | (x, t)|2 o P(x, t) is the probability density. o Immediately implies that sign of wavefunction has no direct physical significance. (x, t) PY 3 P 05

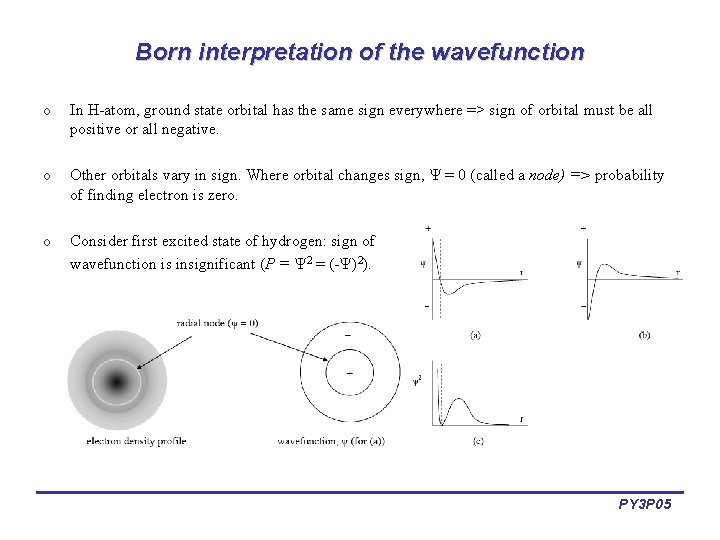
Born interpretation of the wavefunction o In H-atom, ground state orbital has the same sign everywhere => sign of orbital must be all positive or all negative. o Other orbitals vary in sign. Where orbital changes sign, = 0 (called a node) => probability of finding electron is zero. o Consider first excited state of hydrogen: sign of wavefunction is insignificant (P = 2 = (- )2). PY 3 P 05

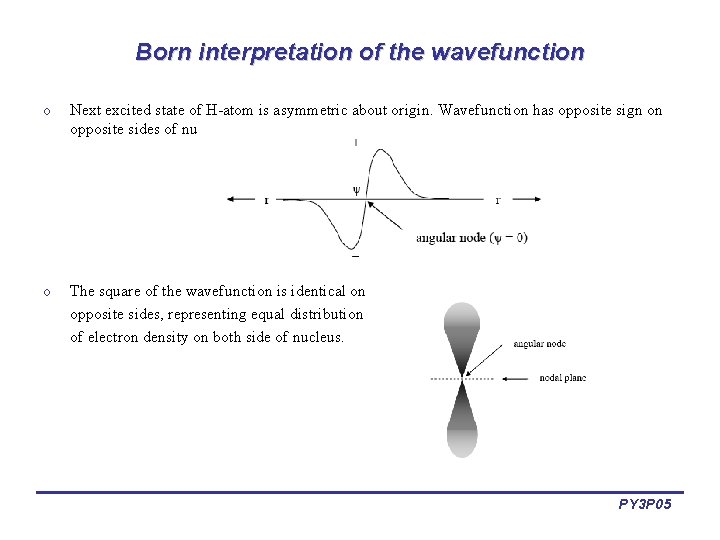
Born interpretation of the wavefunction o Next excited state of H-atom is asymmetric about origin. Wavefunction has opposite sign on opposite sides of nucleus. o The square of the wavefunction is identical on opposite sides, representing equal distribution of electron density on both side of nucleus. PY 3 P 05

Atomic orbitals o Quantum mechanical equivalent of orbits in Bohr model. PY 3 P 05

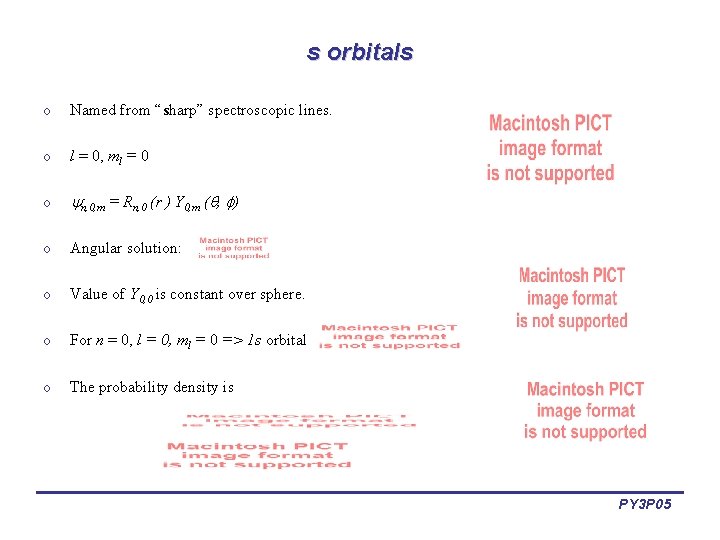
s orbitals o Named from “sharp” spectroscopic lines. o l = 0, ml = 0 o n, 0, m = Rn, 0 (r ) Y 0, m ( , ) o Angular solution: o Value of Y 0, 0 is constant over sphere. o For n = 0, l = 0, ml = 0 => 1 s orbital o The probability density is PY 3 P 05

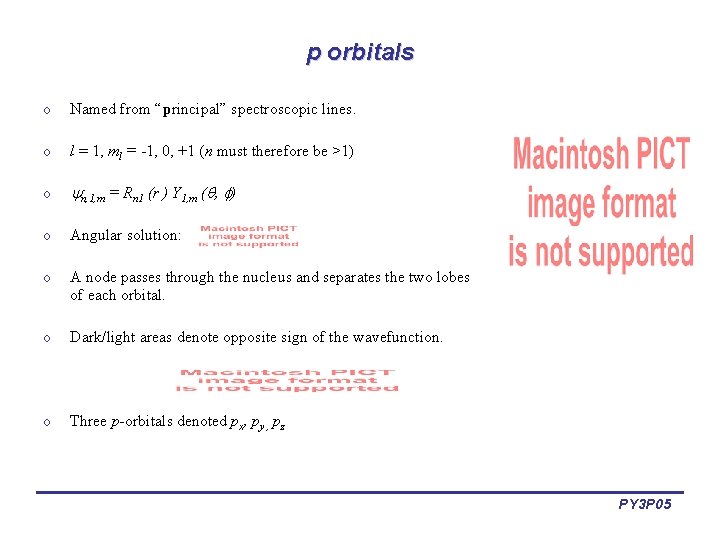
p orbitals o Named from “principal” spectroscopic lines. o l = 1, ml = -1, 0, +1 (n must therefore be >1) o n, 1, m = Rn 1 (r ) Y 1, m ( , ) o Angular solution: o A node passes through the nucleus and separates the two lobes of each orbital. o Dark/light areas denote opposite sign of the wavefunction. o Three p-orbitals denoted px, py , pz PY 3 P 05

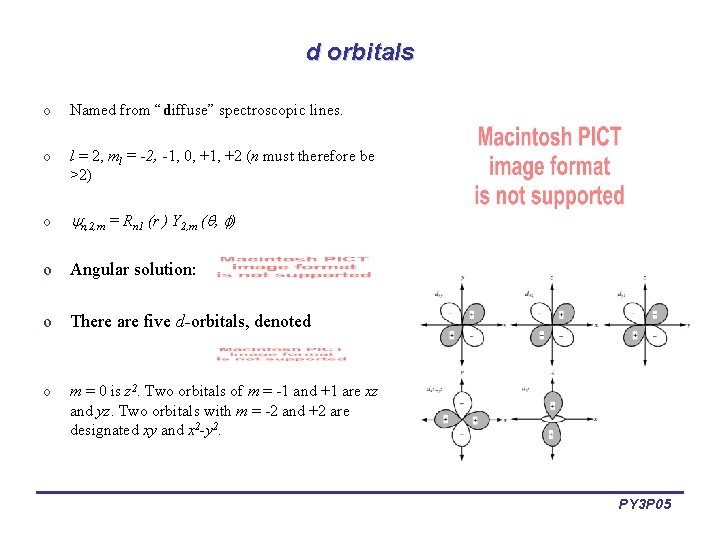
d orbitals o Named from “diffuse” spectroscopic lines. o l = 2, ml = -2, -1, 0, +1, +2 (n must therefore be >2) o n, 2, m = Rn 1 (r ) Y 2, m ( , ) o Angular solution: o There are five d-orbitals, denoted o m = 0 is z 2. Two orbitals of m = -1 and +1 are xz and yz. Two orbitals with m = -2 and +2 are designated xy and x 2 -y 2. PY 3 P 05

Quantum numbers and spectroscopic notation o Angular momentum quantum number: o l = 0 (s subshell) o l = 1 (p subshell) o l = 2 (d subshell) o l = 3 (f subshell) o … o Principal quantum number: o n = 1 (K shell) o n = 2 (L shell) o n = 3 (M shell) o … o If n = 1 and l = 0 = > the state is designated 1 s. n = 3, l = 2 => 3 d state. o Three quantum numbers arise because time-independent Schrödinger equation contains three independent variables, one for each space coordinate. o The eigenvalues of the one-electron atom depend only on n, by the eigenfunctions depend on n, l and ml, since they are the product of Rnl(r ), lml ( ) and ml( ). o For given n, there are generally several values of l and ml => degenerate eigenfunctions. PY 3 P 05

Orbital transitions for hydrogen o Transition between different energy levels of the hydrogenic atom must follow the following selection rules: l = ± 1 m = 0, ± 1 o A Grotrian diagram or a term diagram shows the allowed transitions. o The thicker the line at right, the more probable and hence more intense the transitions. o The intensity of emission/absorption lines could not be explained via Bohr model. PY 3 P 05

Schrödinger vs. Bohr models o Schrodinger’s QM treatment had a number of advantages over semi-classical Bohr model: 1. Probability density orbitals do not violate the Heisenberg Uncertainty Principle. 1. Orbital angular momentum correctly accounted for. 1. Electron spin can be properly treaded. 1. Electron transition rates can be explained. PY 3 P 05