Lecture5 LIPID Fatty acids in food saturated vs













- Slides: 35

Lecture-5 LIPID

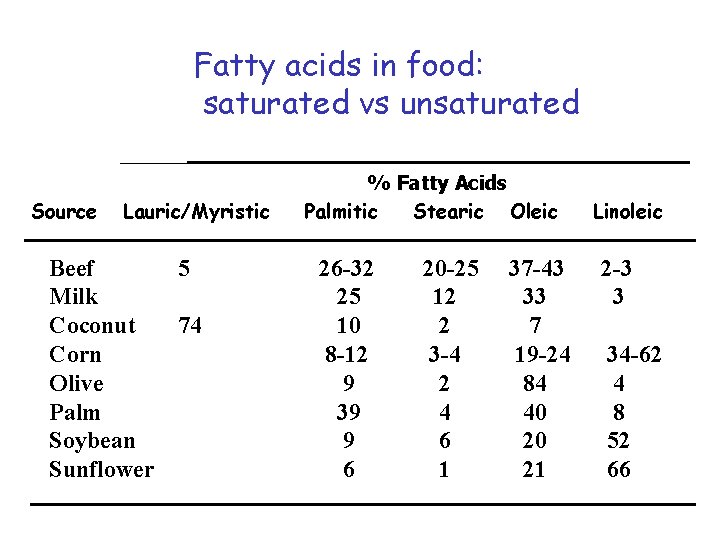
Fatty acids in food: saturated vs unsaturated Source Lauric/Myristic Beef 5 Milk Coconut 74 Corn Olive Palm Soybean Sunflower % Fatty Acids Palmitic Stearic Oleic 26 -32 25 10 8 -12 9 39 9 6 20 -25 12 2 3 -4 2 4 6 1 37 -43 33 7 19 -24 84 40 20 21 Linoleic 2 -3 3 34 -62 4 8 52 66

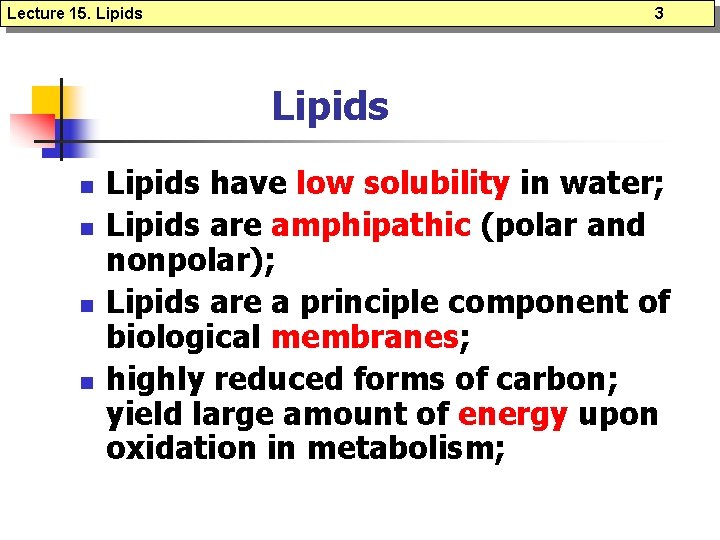
Lecture 15. Lipids 3 Lipids n n Lipids have low solubility in water; Lipids are amphipathic (polar and nonpolar); Lipids are a principle component of biological membranes; highly reduced forms of carbon; yield large amount of energy upon oxidation in metabolism;

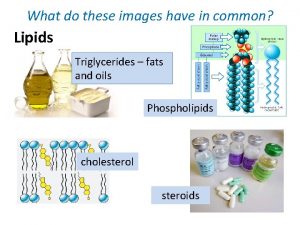
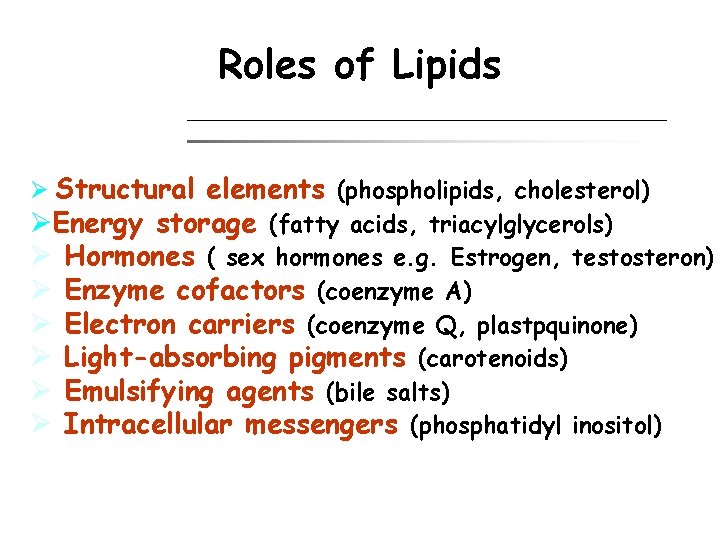
Roles of Lipids Ø Structural elements (phospholipids, cholesterol) ØEnergy storage (fatty acids, triacylglycerols) Ø Hormones ( sex hormones e. g. Estrogen, testosteron) Ø Enzyme cofactors (coenzyme A) Ø Electron carriers (coenzyme Q, plastpquinone) Ø Light-absorbing pigments (carotenoids) Ø Emulsifying agents (bile salts) Ø Intracellular messengers (phosphatidyl inositol)

Spontaneously Formed Lipid Structures • Hydrophobic interactions are important Head group Tail group • Lipid is an amphipathic molecule, but rarely exists as a monomer. air water monolayer Micelle Lipid bilayer

What is soap ? TAG + 3 KOH (Na. OH) 3 RCOO-K+ + glycerol n FA chains can dissolve in oils while charged carboxyl group dissolves in water; n Forms a mixed micelle which can remove oils; n Soap form precipitates with divalent cations (reduces efficiency); n Detergent (modified FAs) do not precipitate with calcium, used as better cleaning agents.

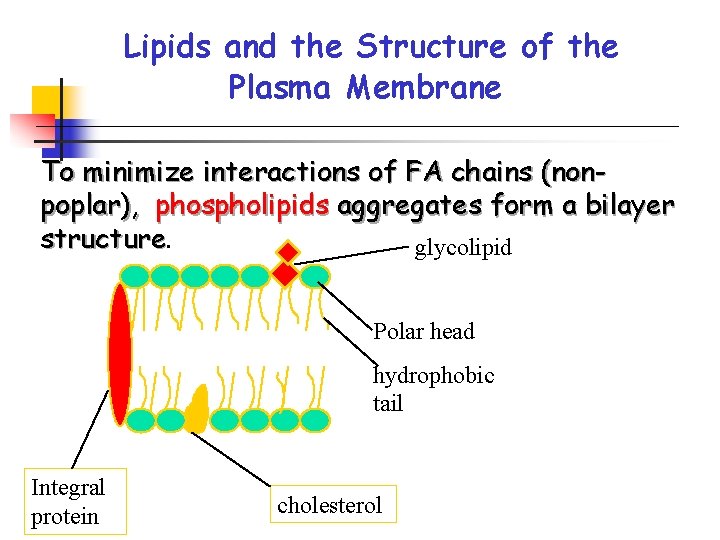
Lipids and the Structure of the Plasma Membrane To minimize interactions of FA chains (nonpoplar), phospholipids aggregates form a bilayer structure. glycolipid Polar head hydrophobic tail Integral protein cholesterol

Fatty Acids as Stored Energy • Fatty acids are the body’s principal form of stored energy • Carbon almost completely reduced as CH 2 • Principal sources: dairy products, meats: Triacylglycerols, phospholipids, sterol esters

Fatty acids (FAs) and nomenclature Structure § Basic formula: CH 3(CH 2)n. COOH § Carboxylic acids with hydrocarbon chains of 4 -24 carbons § Free FAs are found in trace quantities in cells FAs are either: (i) part of a lipid molecule (ii) complexed to a carrier protein (e. g. albumin on blood) § Saturated or unsaturated

Common Fatty Acids n Saturated fatty acids: n n n Lauric acid Myristic acid Palmitic acid Stearic acid 12: 0 14: 0 16: 0 18: 0 Unsaturated fatty acids: n n n Palmitoleic acid Oleic acid Linoleic acid A-linoleic acid G-linoleic acid 16: 1 18: 2 18: 3 (9, 12, 15) 18: 3 (6, 9, 12)

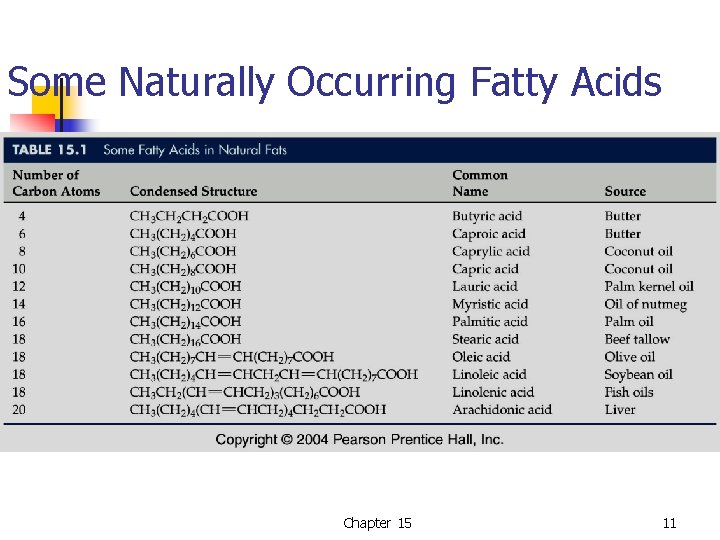
Some Naturally Occurring Fatty Acids Chapter 15 11

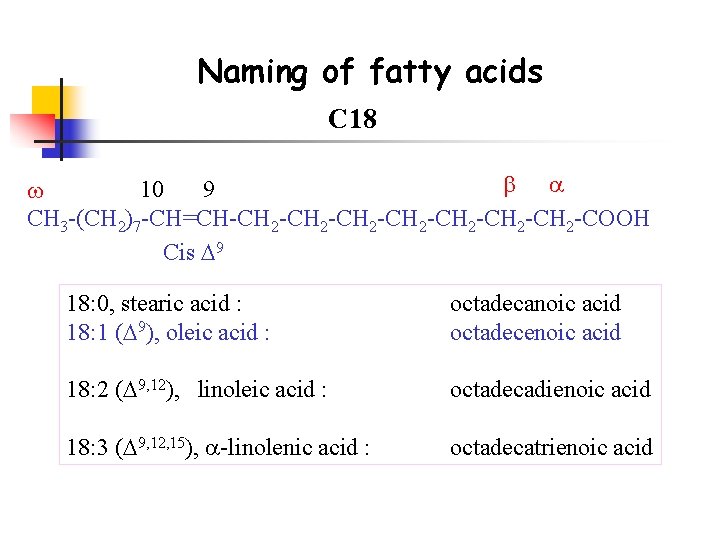
Naming of fatty acids C 18 10 9 CH 3 -(CH 2)7 -CH=CH-CH 2 -CH 2 -CH 2 -COOH Cis 9 18: 0, stearic acid : 18: 1 ( 9), oleic acid : octadecanoic acid octadecenoic acid 18: 2 ( 9, 12), linoleic acid : octadecadienoic acid 18: 3 ( 9, 12, 15), -linolenic acid : octadecatrienoic acid

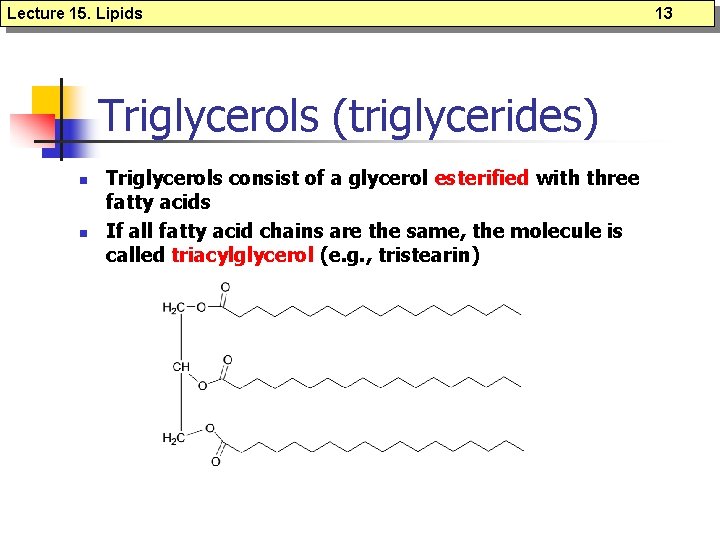
Lecture 15. Lipids Triglycerols (triglycerides) n n Triglycerols consist of a glycerol esterified with three fatty acids If all fatty acid chains are the same, the molecule is called triacylglycerol (e. g. , tristearin) 13

n Fats are largest subgroup of lipids n Made up of fatty acids and glycerol Chapter 15 14

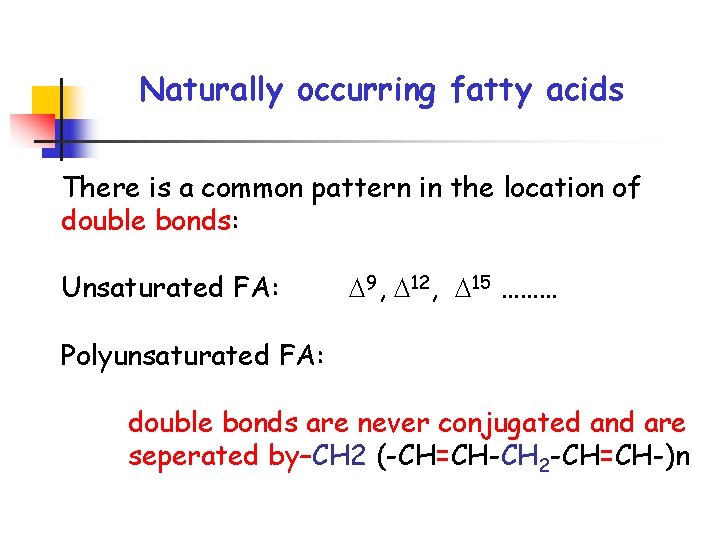
Naturally occurring fatty acids There is a common pattern in the location of double bonds: Unsaturated FA: 9, 12, 15 ……… Polyunsaturated FA: double bonds are never conjugated and are seperated by–CH 2 (-CH=CH-CH 2 -CH=CH-)n

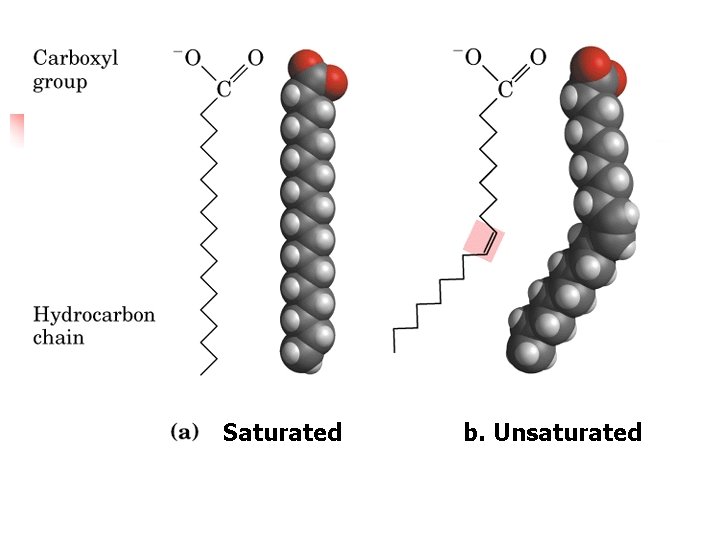
Saturated b. Unsaturated

Lecture 15. Lipids 17 Structural Consequences of Unsaturation n n Saturated chains pack tightly and form more rigid, organized aggregates (i. e. , membranes); Unsaturated chains bend and pack in a less ordered way, with greater potential for motion.

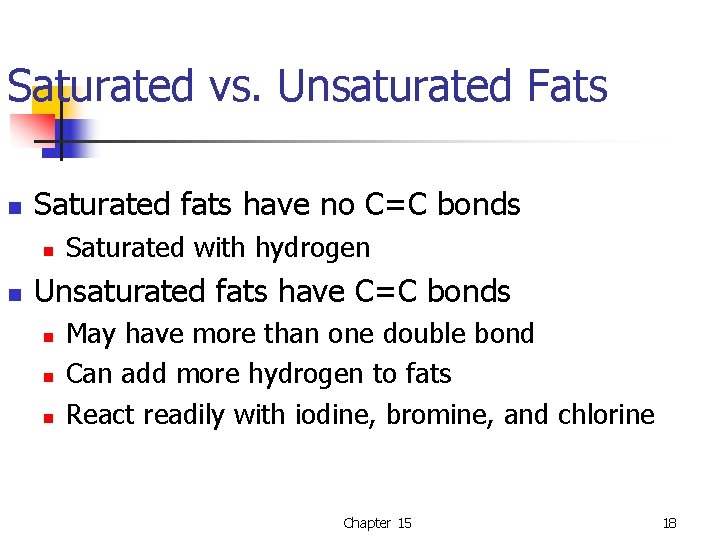
Saturated vs. Unsaturated Fats n Saturated fats have no C=C bonds n n Saturated with hydrogen Unsaturated fats have C=C bonds n n n May have more than one double bond Can add more hydrogen to fats React readily with iodine, bromine, and chlorine Chapter 15 18

Iodine Number n Iodine Number: number of grams of iodine consumed by 100 g of fat Chapter 15 19

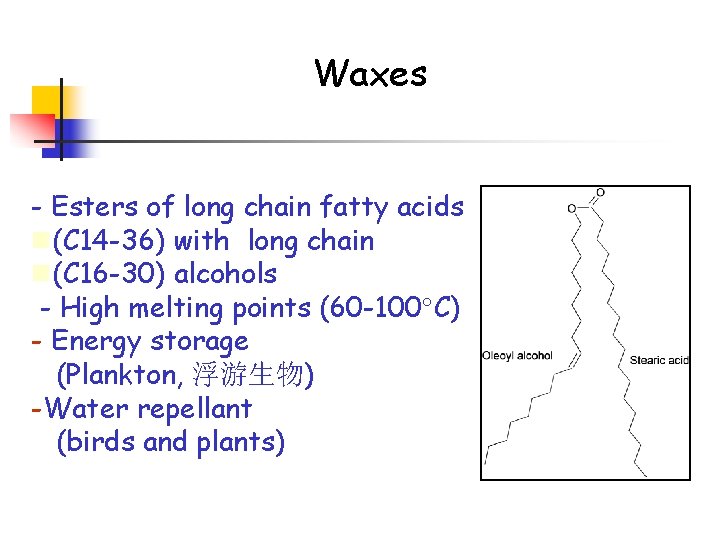
Waxes - Esters of long chain fatty acids n(C 14 -36) with long chain n(C 16 -30) alcohols - High melting points (60 -100 C) - Energy storage (Plankton, 浮游生物) -Water repellant (birds and plants)

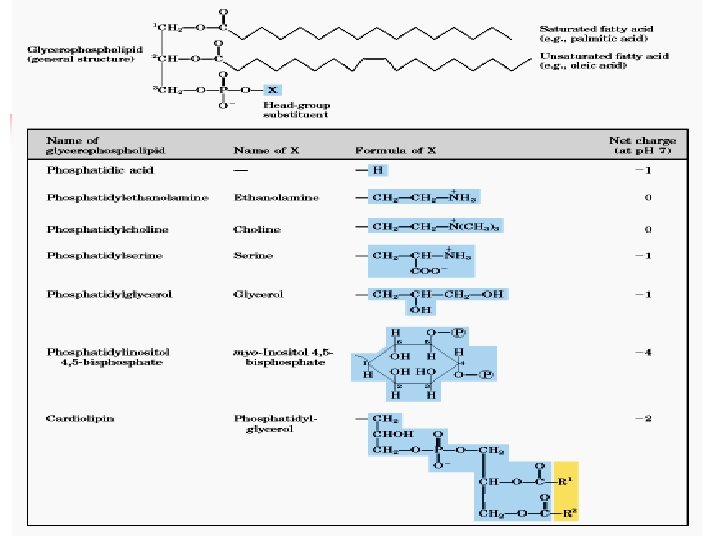
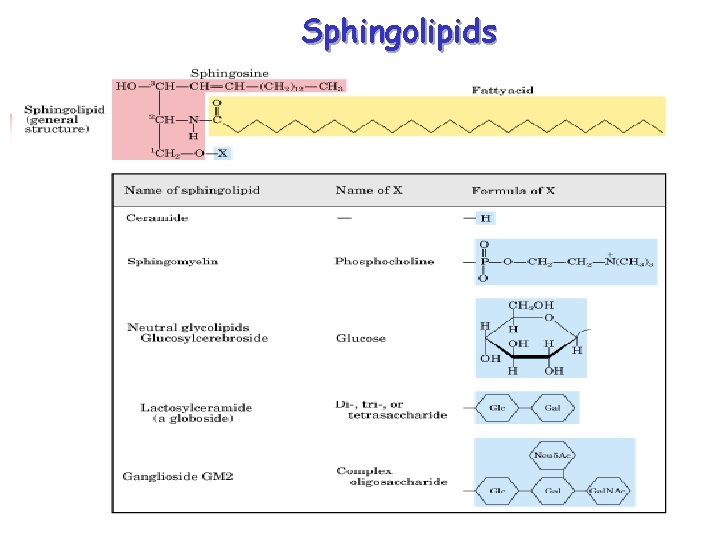
Phospholipids 2 Classes of phospholipids (PL) (i) glycerolphospholipids – glycerol backbone (ii) sphingomyelin – spingosine backbone Glycerolphospholipids - essential for membrane structure - most abundant membrane lipids Sphingolipids - Component of a certain membrane - Sphingosine, fatty acid and glycoside


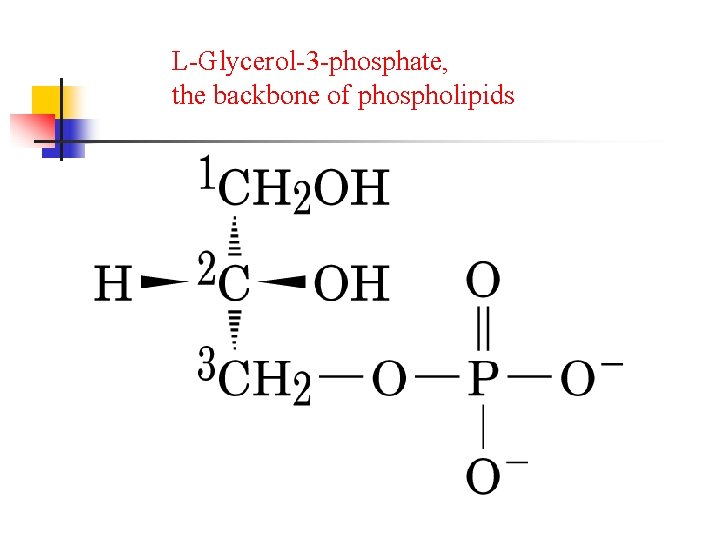
L-Glycerol-3 -phosphate, the backbone of phospholipids


Lecture 15. Lipids 25 Examples of Phosphatides O H 2 C O Phosphatidylcholine O CH CH 3 H 3 C N O O (CH 2)2 O P O CH 3 CH 2 Fatty acid moiety O Phosphatidylethanolamine H 3 N Fatty acid moiety (CH 2)2 Phosphatidylserine COO CH NH 3 H 3 C O Fatty acid moiety

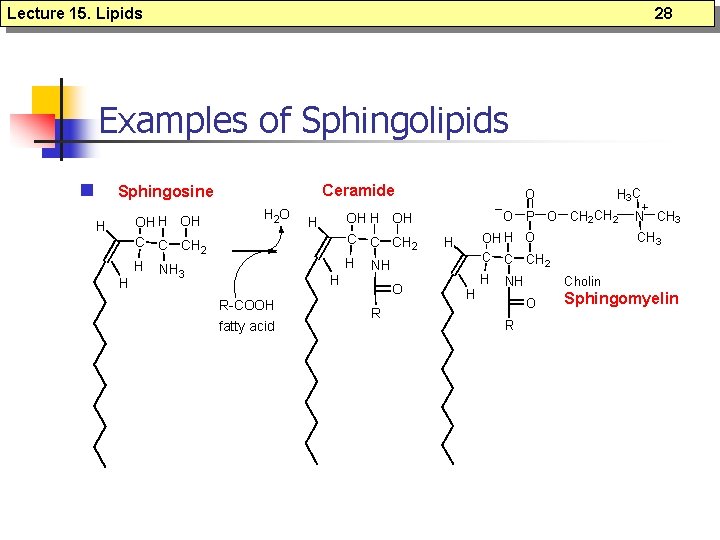
Lecture 15. Lipids 26 Sphingolipids n n n Sphingosine forms the backbone of sphingolipids (rather than glycerol); Sphingosines are important components of biological membranes; Ceramide = sphingosine + fatty acid (via an amide linkage); Sphingomyelins = ceramide + phospholipids (via 1 -hydroxyl group) Glycosphingolipids = ceramide + linked sugar at the 1 -hydroxyl moiety.

Sphingolipids

Lecture 15. Lipids 28 Examples of Sphingolipids n Ceramide Sphingosine H H OH C C CH 2 H NH 3 H 2 O H H R-COOH fatty acid OH H OH C C CH 2 H NH O R H 3 C O O P O CH 2 H H O C C CH 2 H NH O R N CH 3 Cholin Sphingomyelin

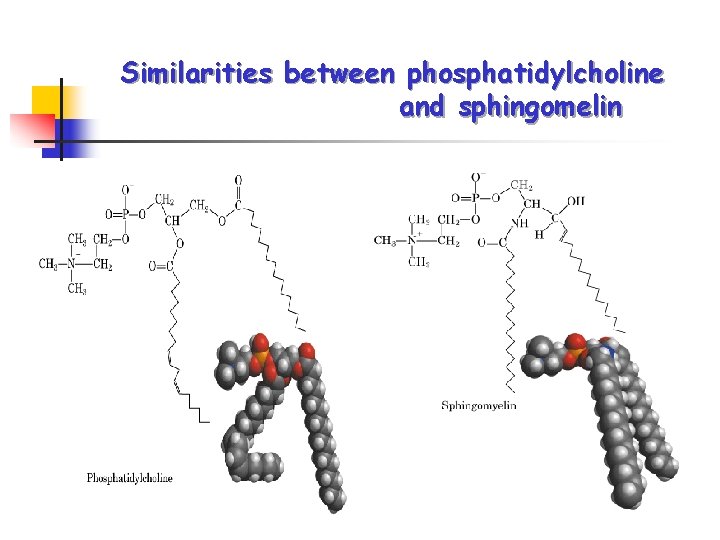
Similarities between phosphatidylcholine and sphingomelin

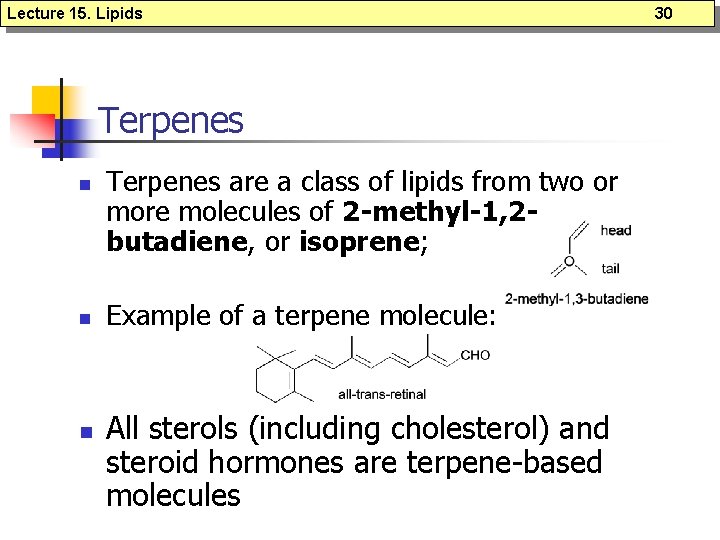
Lecture 15. Lipids Terpenes n n n Terpenes are a class of lipids from two or more molecules of 2 -methyl-1, 2 butadiene, or isoprene; Example of a terpene molecule: All sterols (including cholesterol) and steroid hormones are terpene-based molecules 30

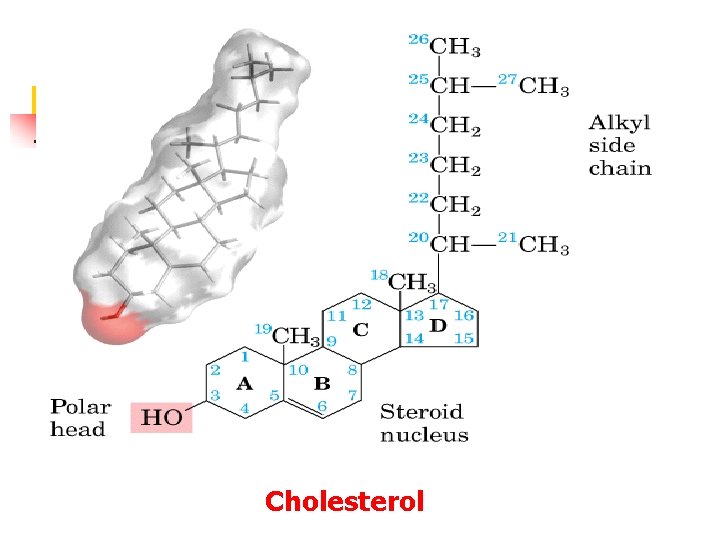
Cholesterol Ø Steroids: (i) cholesterol and sterols of plants and fungi (ii) steroid hormones (iii) bile salts Ø Roles of cholesterol in mammals (i) structural component of plasma membrane and modulates membrane fluidity (ii) precursor of steroid hormones and bile acids Ø Rarely found in plants, never in bacteria

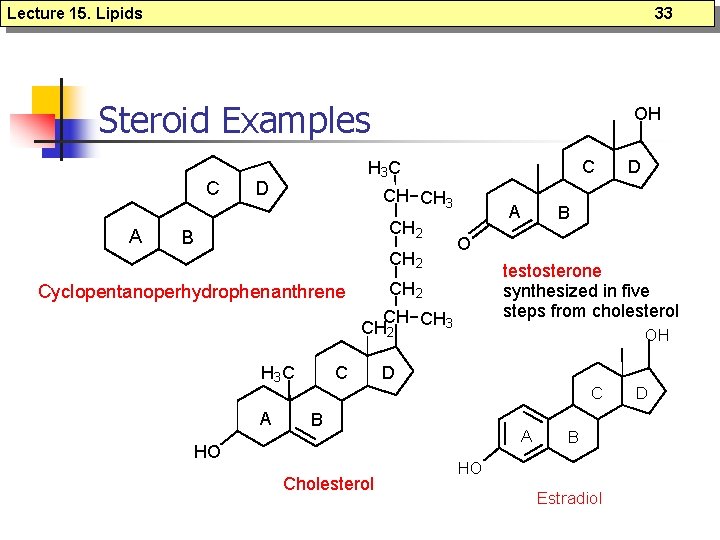
Lecture 15. Lipids Steroids n n n Based on a core structure consisting of three 6 -membered rings and one 5 membered ring, all fused together; Cholesterol is the most common steroid in animals and precursor for all other steroids in animals; Steroid hormones serve many functions in animals - including salt balance, metabolic function and sexual function; 32

Lecture 15. Lipids 33 Steroid Examples C A OH C H 3 C D CH CH 3 CH 2 B CH 2 A testosterone synthesized in five steps from cholesterol CH CH 3 CH 2 H 3 C A C B O CH 2 Cyclopentanoperhydrophenanthrene OH D C B HO Cholesterol D A B HO Estradiol D

Cholesterol

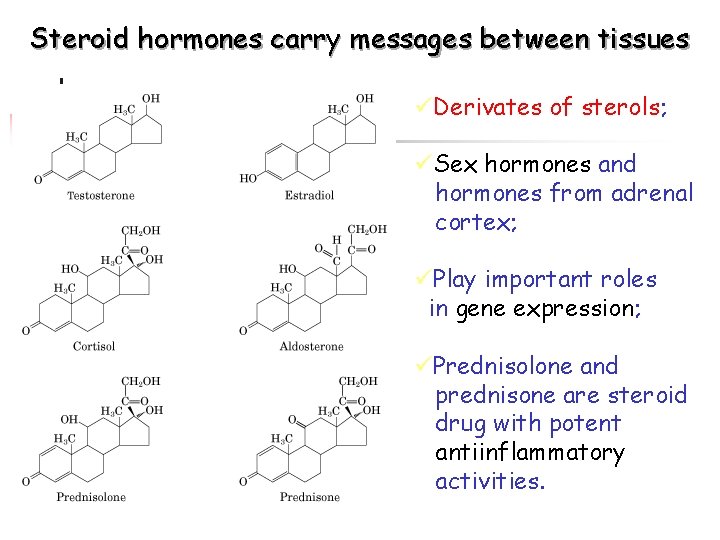
Steroid hormones carry messages between tissues üDerivates of sterols; üSex hormones and hormones from adrenal cortex; üPlay important roles in gene expression; üPrednisolone and prednisone are steroid drug with potent antiinflammatory activities.
 Are fats and lipids the same thing
Are fats and lipids the same thing Saturated fatty acid definition
Saturated fatty acid definition 3,5,8,11,16,19
3,5,8,11,16,19 What are triglycerides
What are triglycerides A saturated fatty acid holds all the hydrogen atoms it can.
A saturated fatty acid holds all the hydrogen atoms it can. Formation of ketone bodies
Formation of ketone bodies Esansiyel yağ asitleri
Esansiyel yağ asitleri Ester bond in fatty acids
Ester bond in fatty acids Essential non essential fatty acids
Essential non essential fatty acids Omega 3 beyin gelişimi
Omega 3 beyin gelişimi Define compound lipids
Define compound lipids Brain capillary
Brain capillary Properties of fatty acids slideshare
Properties of fatty acids slideshare Beta oxidation
Beta oxidation Gluconeogenic amino acids
Gluconeogenic amino acids Doc portal umcg
Doc portal umcg Glycerol and fatty acids
Glycerol and fatty acids Beta oxidation of fatty acids
Beta oxidation of fatty acids Fatty acid oxidation
Fatty acid oxidation Non essential fatty acids
Non essential fatty acids Ester bond in fatty acids
Ester bond in fatty acids Invisible fat examples
Invisible fat examples Activation of fatty acids
Activation of fatty acids Unit 2 food food food
Unit 2 food food food Food chain food chain food chain
Food chain food chain food chain N
N Nucleic acid examples food
Nucleic acid examples food Compression molding method of suppositories
Compression molding method of suppositories Phospholipid fatty acid analysis soil
Phospholipid fatty acid analysis soil Zoloft and microscopic colitis
Zoloft and microscopic colitis Fat deficiency
Fat deficiency Acute fatty liver of pregnancy
Acute fatty liver of pregnancy Fatty appendices
Fatty appendices Involuntary
Involuntary Confluent dentin meaning
Confluent dentin meaning Acg pregnancy
Acg pregnancy