Lecture Two CHEMICAL BONDING Covalent and Coordinate covalent

Lecture Two CHEMICAL BONDING -Covalent and Co-ordinate covalent bonding
COVALENT BONDING • A covalent bond is defined as the force of attraction arising due to mutual sharing of electrons between the two atoms. • The combining atoms may share one, two or three pairs of electrons. • When the two atoms combine by mutual sharing of electrons, then each of the atoms acquires stable configuration of the nearest noble gas

• Involve electron sharing • Usually occurs between two nonmetals • Forms molecular compounds • Two nuclei attract the same shared electrons to form a covalent bond • Orbitals containing the valence electrons overlap to create a common orbital • The electrons are shared by both nuclei
Covalent Compounds • The compounds formed due to covalent bonding are called covalent compounds. • The number of electrons which an atom contributes towards mutual sharing during the formation of a chemical bond is called its covalency in that compound. • Covalency of hydrogen in H 2 (H - H) is 1; that of oxygen in O 2 is 2 (O = O), and that of nitrogen in N 2 is three (N º N)
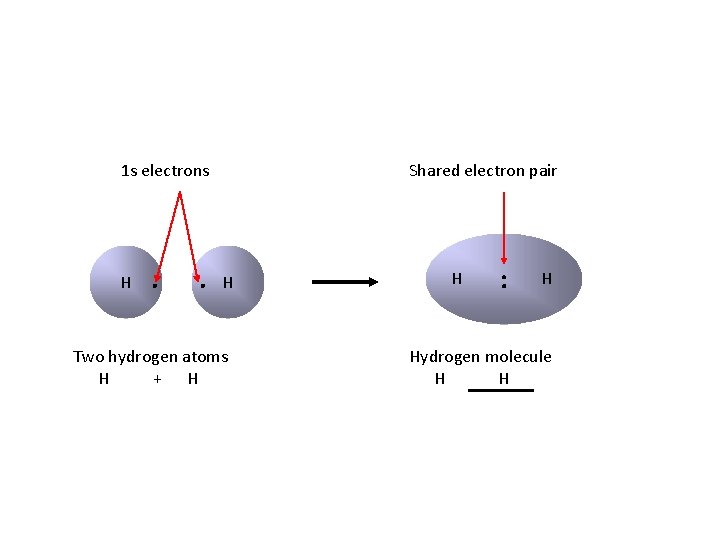
1 s electrons H ∙ ∙ Shared electron pair H Two hydrogen atoms H + H H : H Hydrogen molecule H H
Types of electrons in covalent bonding Bonding Electrons - The pairs of valence electrons involved in the covalent bond formation Nonbonding Electrons (Lone Pairs of Electrons) - The pairs of valence electrons not involved in electron sharing
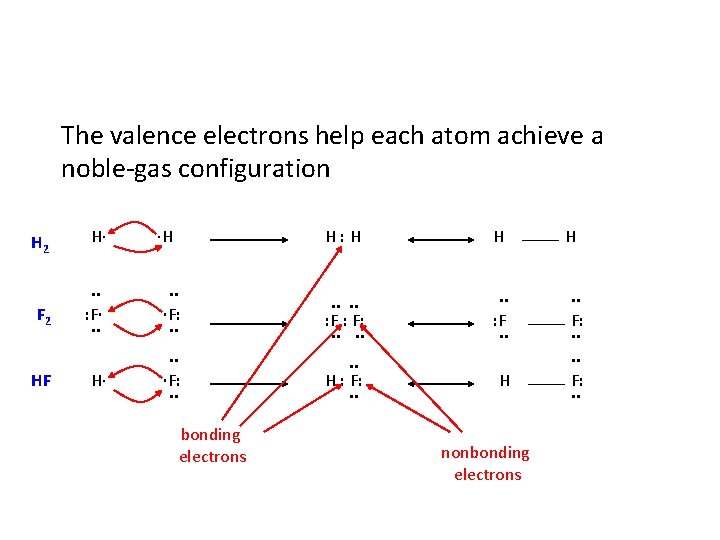
The valence electrons help each atom achieve a noble-gas configuration H 2 H∙ F 2 . . : F∙. . H∙ HF ∙H H: H H . . ∙F: . . . : F. . ∙F: . . H : F: . . H bonding electrons nonbonding electrons H. . F: . .

Characteristics of a Covalent Bond • Mode of formation. Covalent bonds are formed due to mutual sharing of one or more pairs of electrons. • Directional character. Covalent bonds are directional in nature. This is because in a covalent bond, the shared pair of electrons remains localised in a definite space between the nuclei of the two atoms. This gives a directional character to the covalent bond.
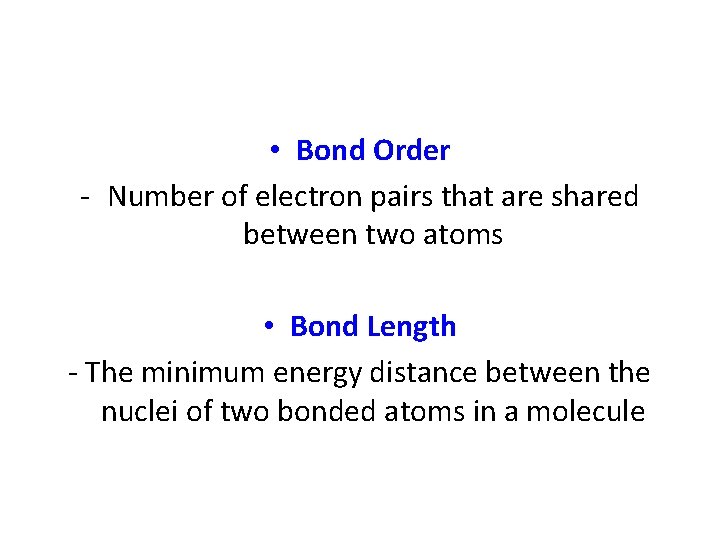
• Bond Order - Number of electron pairs that are shared between two atoms • Bond Length - The minimum energy distance between the nuclei of two bonded atoms in a molecule
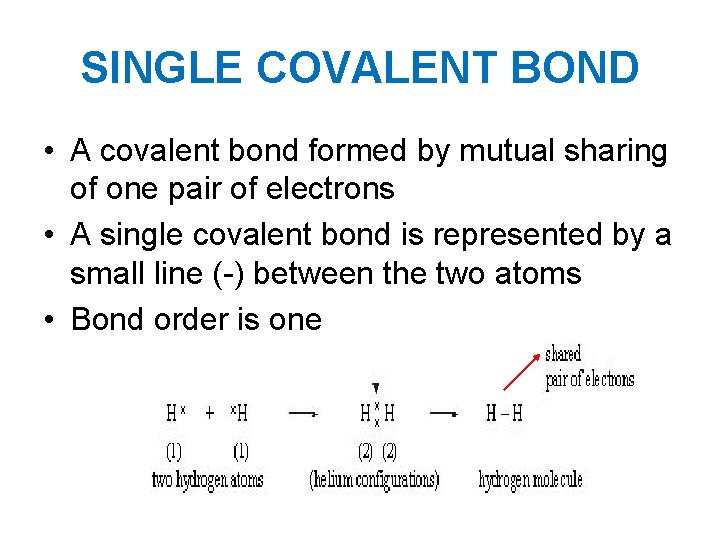
SINGLE COVALENT BOND • A covalent bond formed by mutual sharing of one pair of electrons • A single covalent bond is represented by a small line (-) between the two atoms • Bond order is one
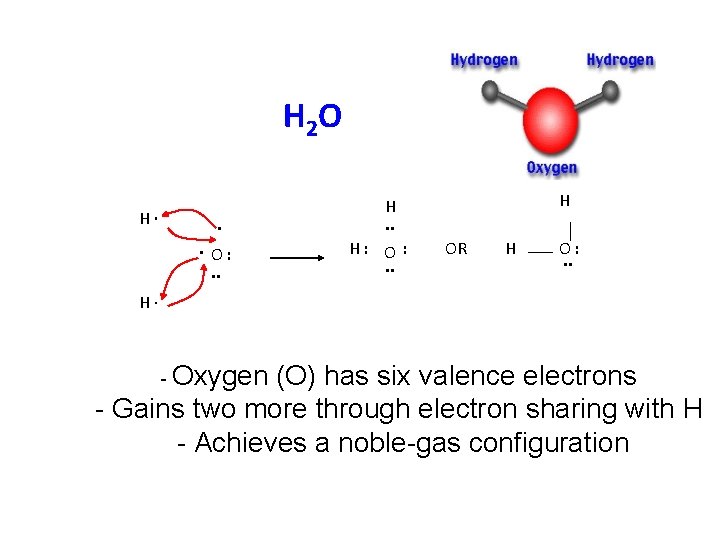
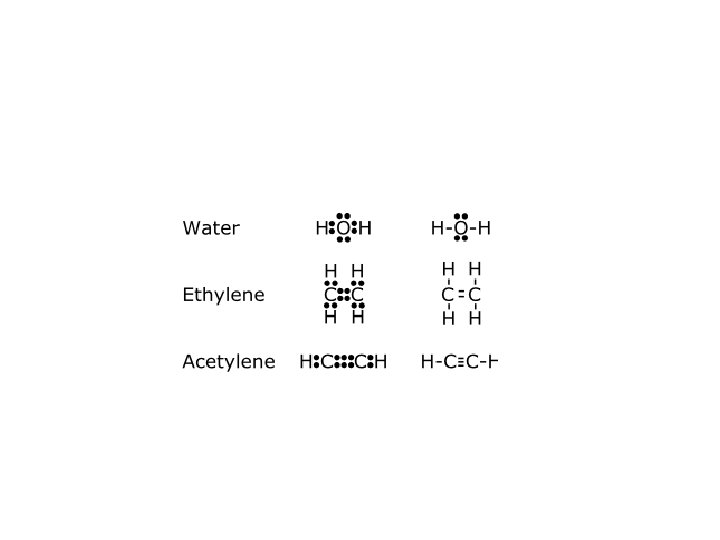
H 2 O H∙ . . O: . . H H. . H: O : . . OR H O: . . H∙ - Oxygen (O) has six valence electrons - Gains two more through electron sharing with H - Achieves a noble-gas configuration
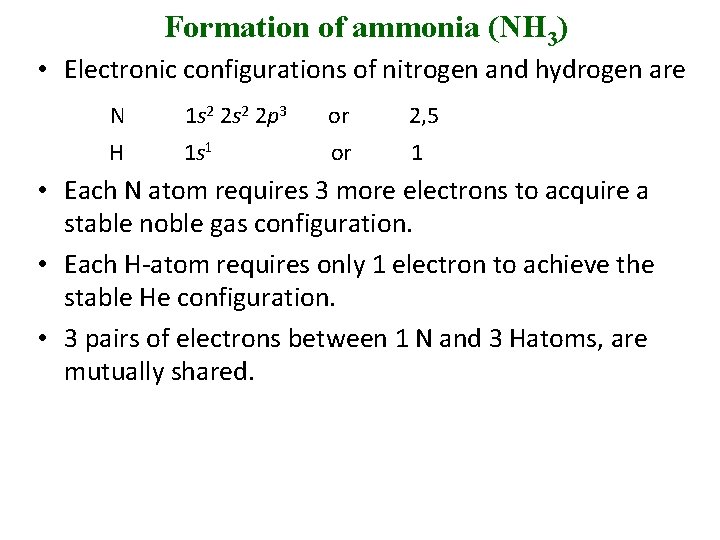
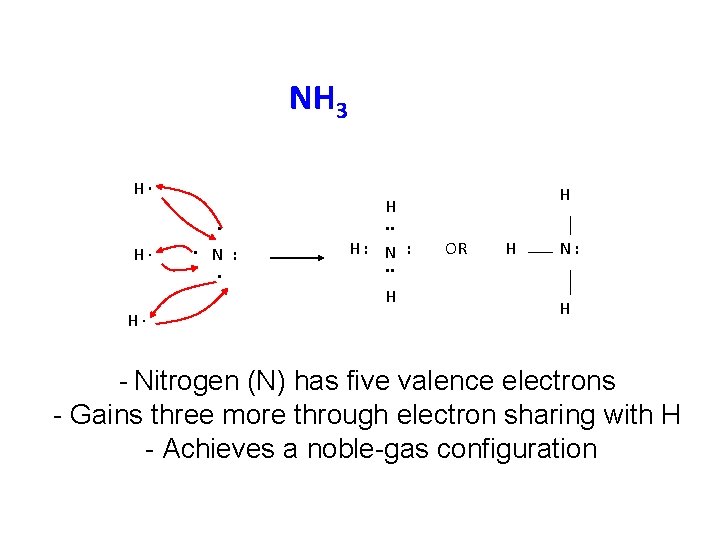
Formation of ammonia (NH 3) • Electronic configurations of nitrogen and hydrogen are N 1 s 2 2 p 3 or 2, 5 H 1 s 1 or 1 • Each N atom requires 3 more electrons to acquire a stable noble gas configuration. • Each H-atom requires only 1 electron to achieve the stable He configuration. • 3 pairs of electrons between 1 N and 3 Hatoms, are mutually shared.

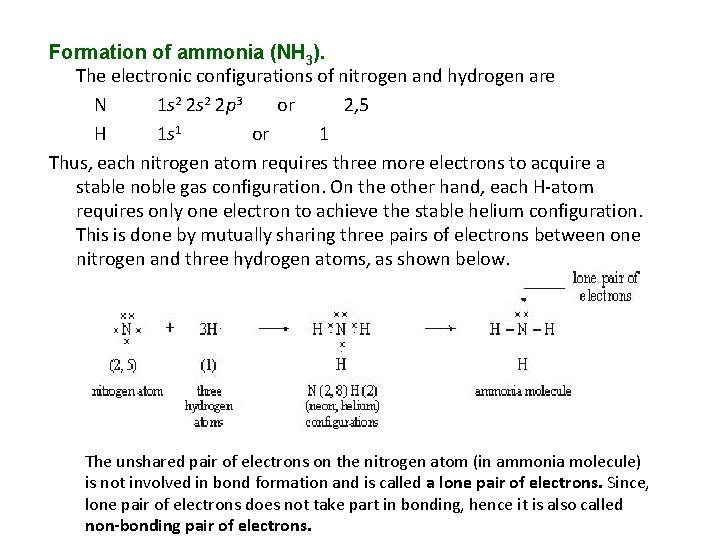
Formation of ammonia (NH 3). The electronic configurations of nitrogen and hydrogen are N 1 s 2 2 p 3 or 2, 5 H 1 s 1 or 1 Thus, each nitrogen atom requires three more electrons to acquire a stable noble gas configuration. On the other hand, each H-atom requires only one electron to achieve the stable helium configuration. This is done by mutually sharing three pairs of electrons between one nitrogen and three hydrogen atoms, as shown below. The unshared pair of electrons on the nitrogen atom (in ammonia molecule) is not involved in bond formation and is called a lone pair of electrons. Since, lone pair of electrons does not take part in bonding, hence it is also called non-bonding pair of electrons.
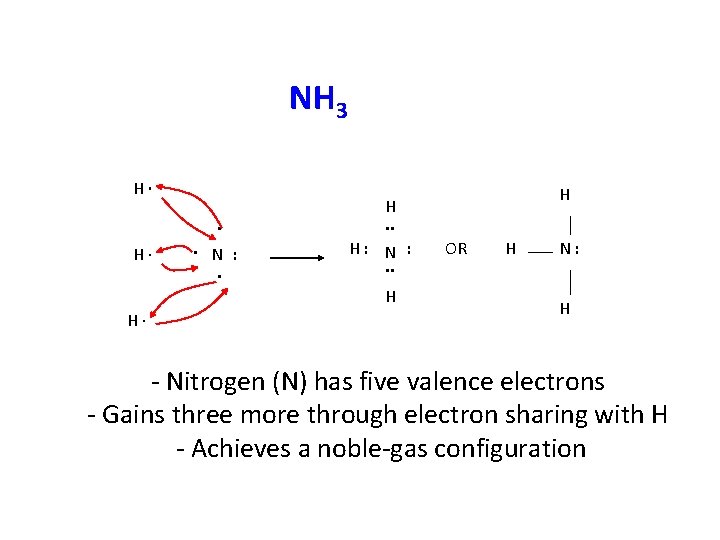
NH 3 H∙. H∙ . N : . H: N : . . H H∙ H H. . OR H N: H - Nitrogen (N) has five valence electrons - Gains three more through electron sharing with H - Achieves a noble-gas configuration
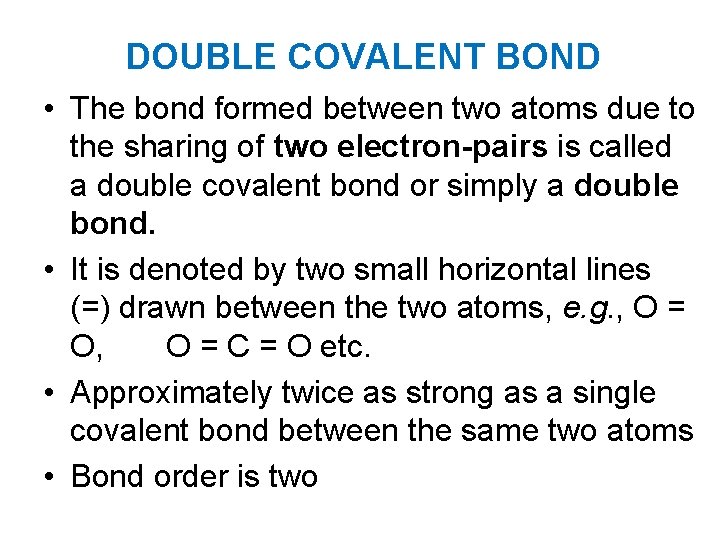
DOUBLE COVALENT BOND • The bond formed between two atoms due to the sharing of two electron-pairs is called a double covalent bond or simply a double bond. • It is denoted by two small horizontal lines (=) drawn between the two atoms, e. g. , O = O, O = C = O etc. • Approximately twice as strong as a single covalent bond between the same two atoms • Bond order is two
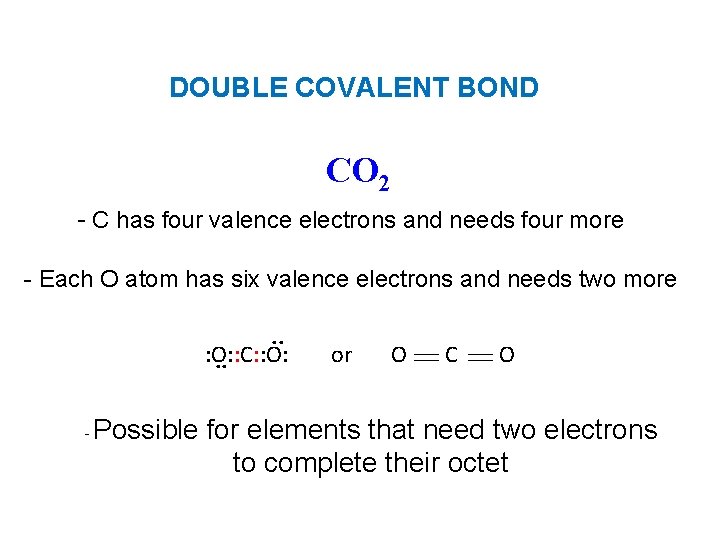
DOUBLE COVALENT BOND CO 2 - C has four valence electrons and needs four more - Each O atom has six valence electrons and needs two more. . : O: : C: : O: . . - Possible or O C O for elements that need two electrons to complete their octet
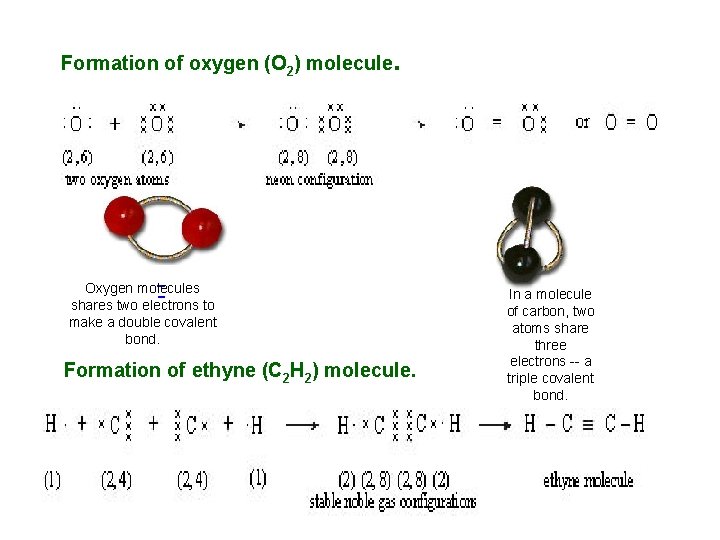
Formation of oxygen (O 2) molecule . Oxygen molecules shares two electrons to make a double covalent bond. Formation of ethyne (C 2 H 2) molecule. In a molecule of carbon, two atoms share three electrons -- a triple covalent bond.
TRIPLE COVALENT BOND • Two atoms share three pairs of valence electrons • Bond formed due to the sharing of 3 electronpairs is called a triple covalent bond or a triple bond. • Three small horizontal lines between the two atoms denote a triple bond, e. g. , N º N, and H - C º C- H • Approximately thrice as strong as a single covalent bond between the same two atoms • Bond order is three • Bond length decreases with increasing bond order
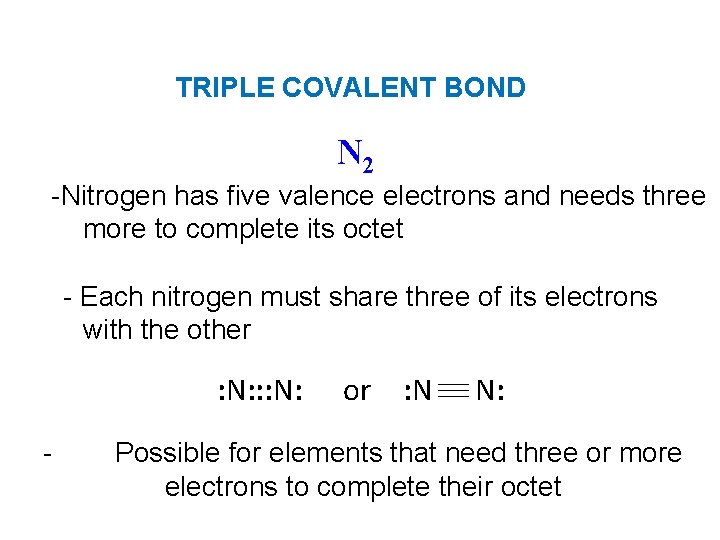
TRIPLE COVALENT BOND N 2 -Nitrogen has five valence electrons and needs three more to complete its octet - Each nitrogen must share three of its electrons with the other : N: : : N: - or : N N: Possible for elements that need three or more electrons to complete their octet
NH 3 H∙. H∙ . N : . H: N : . . H H∙ H H. . OR H N: H - Nitrogen (N) has five valence electrons - Gains three more through electron sharing with H - Achieves a noble-gas configuration
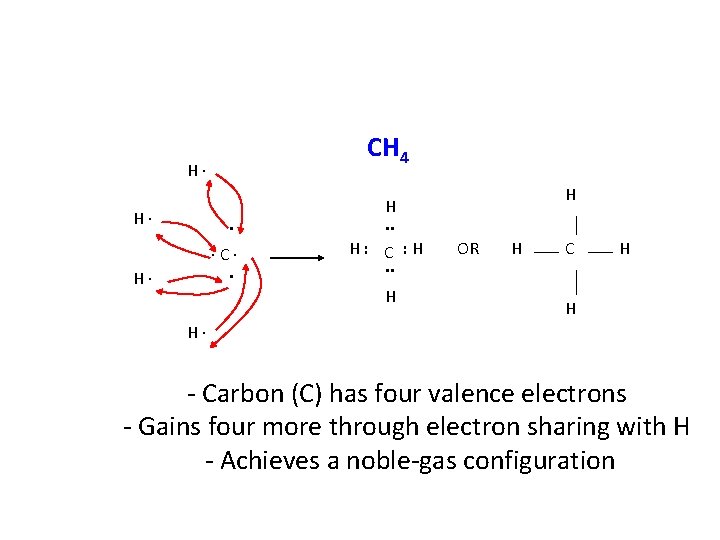
CH 4 H∙ H∙ ∙C∙. H H. . H: C : H. . H OR H C H H H∙ - Carbon (C) has four valence electrons - Gains four more through electron sharing with H - Achieves a noble-gas configuration
Comparison Between Single, Double and Triple Covalent Bonds • Single bond is formed by the sharing of 1 electron pair, (2 -electrons), double bond is formed by the sharing of 2 electron pairs, (4 - electrons), whereas a triple bond involves sharing of 3 electron pairs, (6 electrons). – In a triple bond, 6 electrons attract the nuclei with greater force. This decreases the distance of separation between the two nuclei. – In a double bond, 4 electrons attract the nuclei with a relatively lesser force, – In a single bond, 2 electrons hold the nuclei with a still lesser force. • Therefore, the bond lengths follow the order, Triple bond length < Double bond length < Single bond length
• Since, a shorter bond means greater bond strength hence, the energy required to separate the bonded atoms (called bond energy) follows the order, Triple bond > Double bond > Single bond
Formation of a covalent bond • Formation of a covalent bond is favoured by, (i) High ionisation enthalpy (or energy) of the combining elements. (ii) Nearly equal electron gain enthalpies (or electron affinities) and equal electronegativities of the combining elements. (iii) High nuclear charge and small atomic size of the combining elements.
COORDINATE COVALENT BOND • Coordinate bond is formed when the shared electron-pair is provided by one of the combining atoms. The atom which provides the electronpair is termed as the donor atom, while the other atom which accepts it, is termed as the acceptor atom • The bond formed when one-sided sharing of electrons take place is called a coordinate bond. Such a bond is also known as dative bond. A coordinate bond is represented by an arrow (®) pointing towards the acceptor atom.
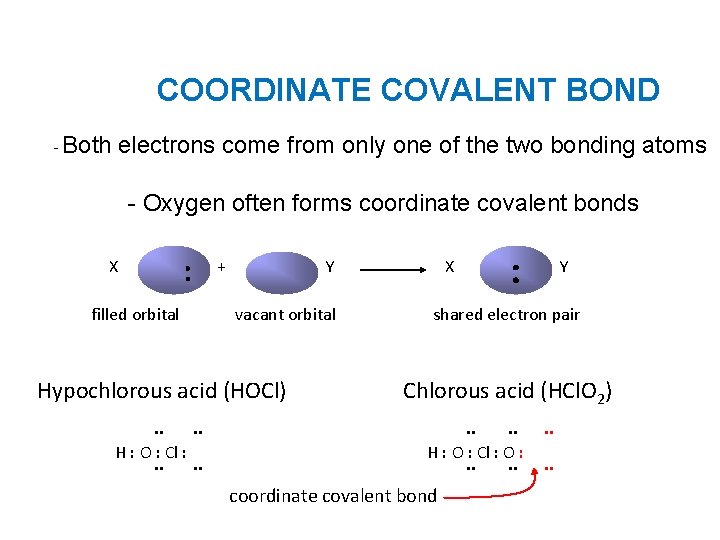
COORDINATE COVALENT BOND - Both electrons come from only one of the two bonding atoms - Oxygen often forms coordinate covalent bonds X : filled orbital + Y vacant orbital Hypochlorous acid (HOCl). . H : O : Cl : . . X : Y shared electron pair Chlorous acid (HCl. O 2). . H : O : Cl : O : . . coordinate covalent bond . .
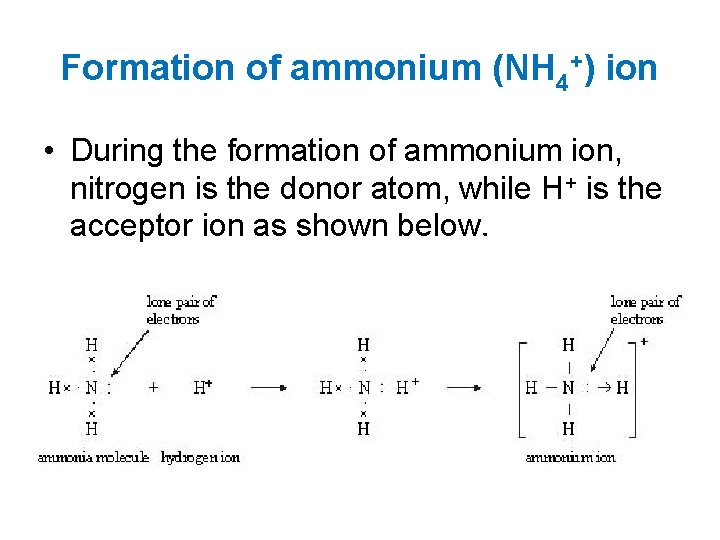
Formation of ammonium (NH 4+) ion • During the formation of ammonium ion, nitrogen is the donor atom, while H+ is the acceptor ion as shown below.
- Slides: 29