Lecture Presentation Unit 7 Day 6 Chemical Equilibrium

Lecture Presentation Unit 7 Day 6 Chemical Equilibrium James F. Kirby Quinnipiac University Hamden, CT Edited by M. Day © 2015 Pearson Education, Inc.

Warm Up TAKE OUT: Lab Notebook and Lab Handout MAKE NEW SECTION: Intro Activity Results WORK TOGETHER: With Lab Partner to “Analyze the Results” TIME: 12 MINUTES WHEN DONE: Share which Lab Station you plan to start at (A, C, D, or E) Equilibrium © 2015 Pearson Education, Inc.

Agenda • COMPLETE: All 4 Lab Stations • Homework: Explanation AND AP Review Questions • Use Background section of lab, class website, internet resources, and remind. com) Equilibrium © 2015 Pearson Education, Inc.

Lab Report: Procedure, Data, Discussion of Theory A. HIn (aq) ⇌ H+ (aq) + ln-(aq) H+(aq) + OH-(aq) H 2 O(l) Procedure Observations Explanation Initial color of water and bromothymol blue Add 0. 1 M HCl Add 0. 1 M Na. OH Additional drops of HCl and Na. OH © 2015 Pearson Education, Inc. Equilibrium
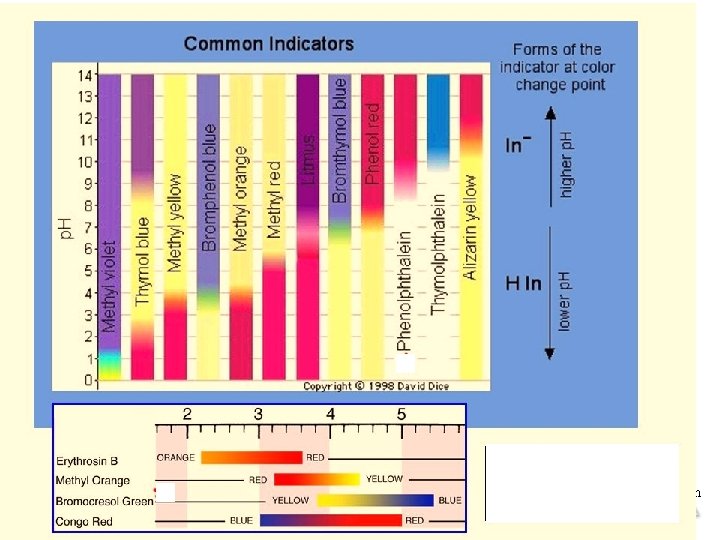
Equilibrium © 2015 Pearson Education, Inc.

Equilibrium © 2015 Pearson Education, Inc.
![Lab Report: Procedure, Data, Discussion of Theory C. [Co(H 2 O)6]2+(aq) + 4 HCl Lab Report: Procedure, Data, Discussion of Theory C. [Co(H 2 O)6]2+(aq) + 4 HCl](http://slidetodoc.com/presentation_image_h2/03d27eed705214b3d638c8b88b65ea9d/image-7.jpg)
Lab Report: Procedure, Data, Discussion of Theory C. [Co(H 2 O)6]2+(aq) + 4 HCl (aq) + Heat ⇌ [Co. Cl 4]2 -(aq) + 6 H 2 O(l) Ag+(aq) + Cl-(aq) Ag. Cl(s) Add Cobalt Complex Ion Color Information Somewhere on Procedure Observations Explanation Add 6. 0 M HCl drops (tube A) Add 0. 1 M Ag. NO 3 drops (tube B) Add distilled water drops (tube C) Add 5 -6 grains Ca. Cl 2 to tube C Test tube C placed in ice water bath for 2 -3 minutes Test tube C placed in hot bath for 2 -3 Education, mins Inc. © 2015 Pearson Equilibrium
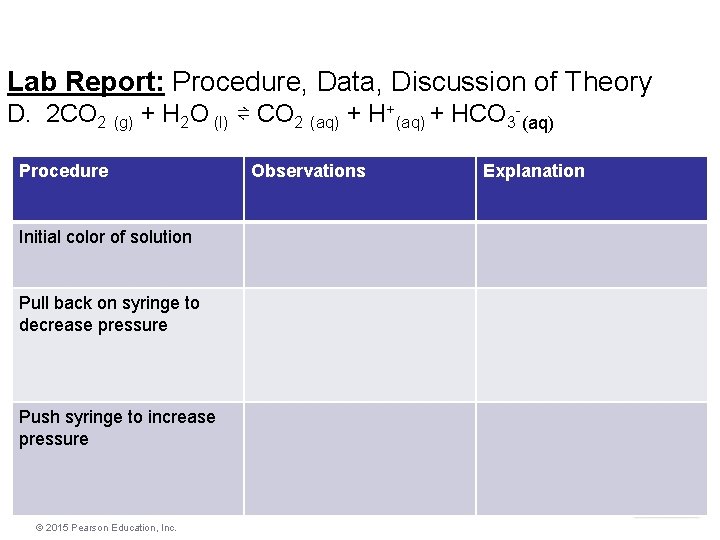
Lab Report: Procedure, Data, Discussion of Theory D. 2 CO 2 (g) + H 2 O (l) ⇌ CO 2 (aq) + H+(aq) + HCO 3 -(aq) Procedure Observations Explanation Initial color of solution Pull back on syringe to decrease pressure Push syringe to increase pressure Equilibrium © 2015 Pearson Education, Inc.
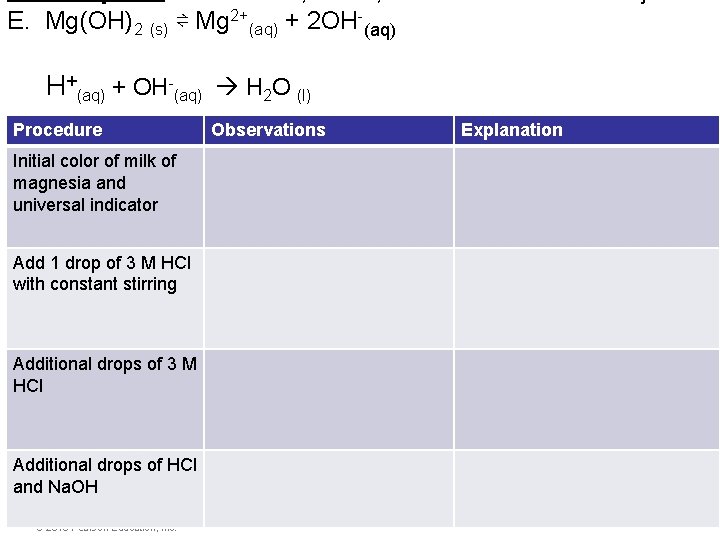
E. Mg(OH)2 (s) ⇌ Mg 2+(aq) + 2 OH-(aq) H+(aq) + OH-(aq) H 2 O (l) Procedure Observations Explanation Initial color of milk of magnesia and universal indicator Add 1 drop of 3 M HCl with constant stirring Additional drops of 3 M HCl Additional drops of HCl and Na. OH © 2015 Pearson Education, Inc. Equilibrium

Clean Up for the Next Group! Equilibrium © 2015 Pearson Education, Inc.

Applications of Le. Chatelier’s Principle COMPLETE: All 4 lab stations WRITE: Observations (Explanation is homework) TIME: 10 -12 minutes per station WHEN DONE: CLEAN UP FOR NEXT GROUP Equilibrium © 2015 Pearson Education, Inc.
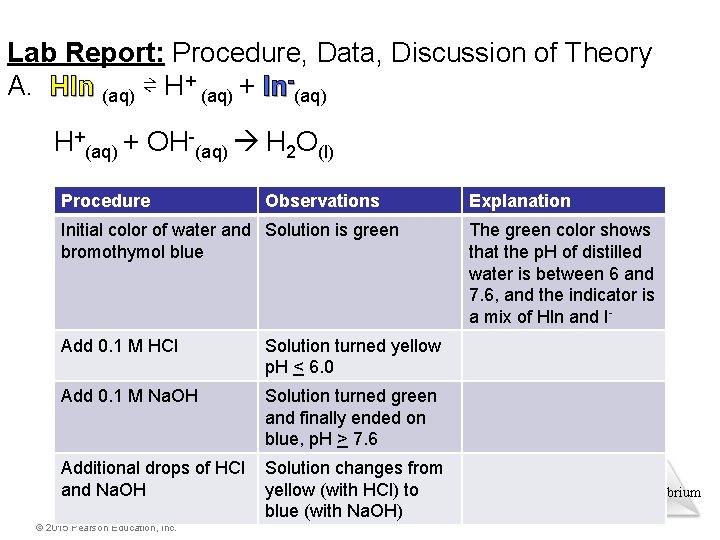
Lab Report: Procedure, Data, Discussion of Theory A. HIn (aq) ⇌ H+ (aq) + ln-(aq) H+(aq) + OH-(aq) H 2 O(l) Procedure Observations Initial color of water and Solution is green bromothymol blue Add 0. 1 M HCl Solution turned yellow p. H < 6. 0 Add 0. 1 M Na. OH Solution turned green and finally ended on blue, p. H > 7. 6 Additional drops of HCl and Na. OH Solution changes from yellow (with HCl) to blue (with Na. OH) © 2015 Pearson Education, Inc. Explanation The green color shows that the p. H of distilled water is between 6 and 7. 6, and the indicator is a mix of Hln and I- Equilibrium
![Lab Report: Procedure, Data, Discussion of Theory C. [Co(H 2 O)6]2+(aq) + 4 HCl Lab Report: Procedure, Data, Discussion of Theory C. [Co(H 2 O)6]2+(aq) + 4 HCl](http://slidetodoc.com/presentation_image_h2/03d27eed705214b3d638c8b88b65ea9d/image-13.jpg)
Lab Report: Procedure, Data, Discussion of Theory C. [Co(H 2 O)6]2+(aq) + 4 HCl (aq) + Heat ⇌ [Co. Cl 4]2 -(aq) + 6 H 2 O(l) Ag+(aq) + Cl-(aq) Ag. Cl(s) Procedure Observations Add 6. 0 M HCl drops (tube A) Solution turned blue Add 0. 1 M Ag. NO 3 drops (tube B) White solid precipitate and solution turned pink Add distilled water drops (tube C) Solution turned pink Add 5 -6 grains Ca. Cl 2 to tube C Crystals dissolved and solution turned blue Test tube C placed in ice water bath for 2 -3 minutes Solution turned pink Test tube C placed in hot Solution turned blue bath for 2 -3 mins © 2015 Pearson Education, Inc. Explanation Equilibrium
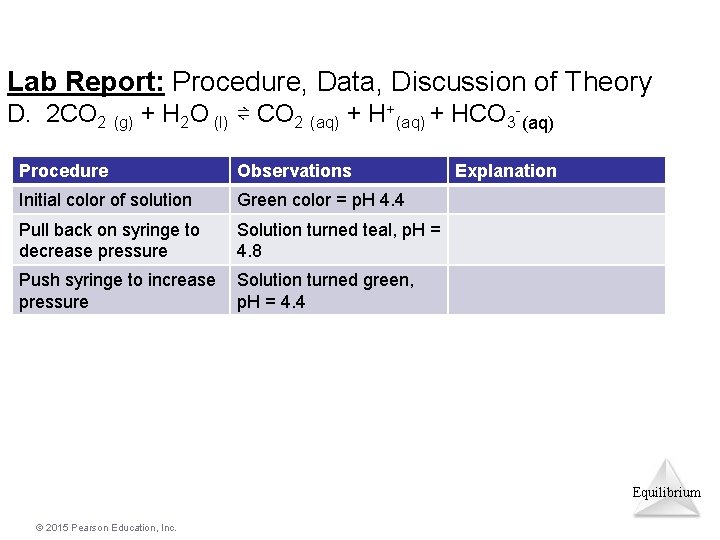
Lab Report: Procedure, Data, Discussion of Theory D. 2 CO 2 (g) + H 2 O (l) ⇌ CO 2 (aq) + H+(aq) + HCO 3 -(aq) Procedure Observations Initial color of solution Green color = p. H 4. 4 Pull back on syringe to decrease pressure Solution turned teal, p. H = 4. 8 Push syringe to increase pressure Solution turned green, p. H = 4. 4 Explanation Equilibrium © 2015 Pearson Education, Inc.
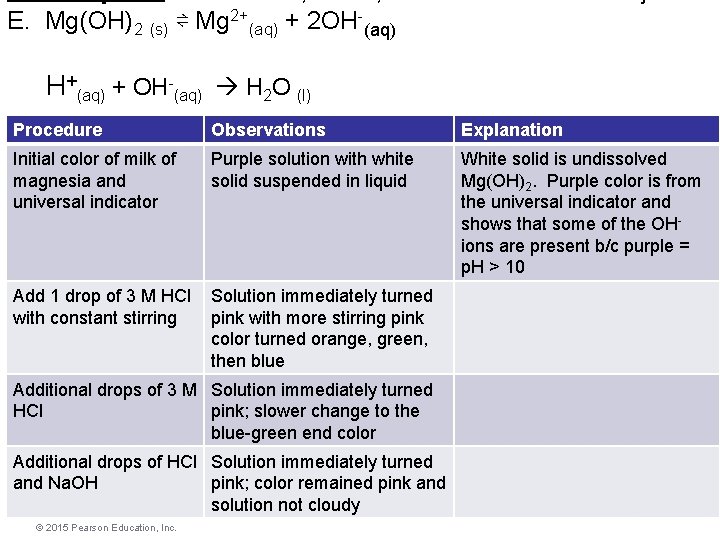
E. Mg(OH)2 (s) ⇌ Mg 2+(aq) + 2 OH-(aq) H+(aq) + OH-(aq) H 2 O (l) Procedure Observations Explanation Initial color of milk of magnesia and universal indicator Purple solution with white solid suspended in liquid White solid is undissolved Mg(OH)2. Purple color is from the universal indicator and shows that some of the OHions are present b/c purple = p. H > 10 Add 1 drop of 3 M HCl with constant stirring Solution immediately turned pink with more stirring pink color turned orange, green, then blue Additional drops of 3 M Solution immediately turned HCl pink; slower change to the blue-green end color Additional drops of HCl Solution immediately turned and Na. OH pink; color remained pink and solution not cloudy © 2015 Pearson Education, Inc. Equilibrium
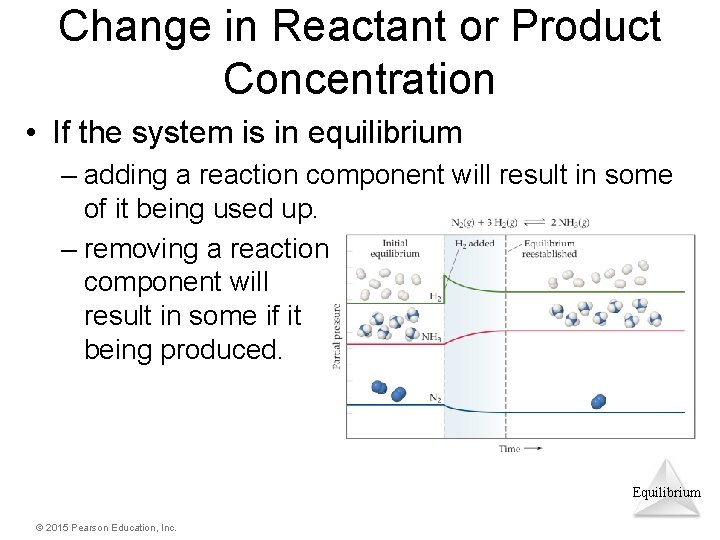
Change in Reactant or Product Concentration • If the system is in equilibrium – adding a reaction component will result in some of it being used up. – removing a reaction component will result in some if it being produced. Equilibrium © 2015 Pearson Education, Inc.
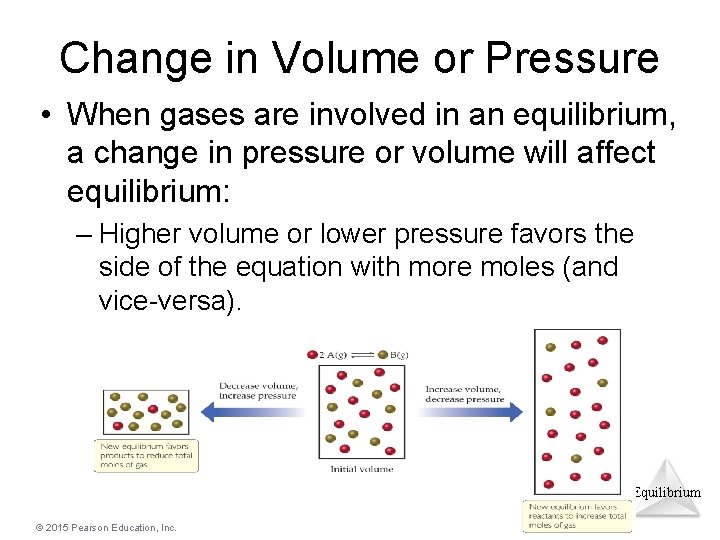
Change in Volume or Pressure • When gases are involved in an equilibrium, a change in pressure or volume will affect equilibrium: – Higher volume or lower pressure favors the side of the equation with more moles (and vice-versa). Equilibrium © 2015 Pearson Education, Inc.
Change in Temperature • Is the reaction endothermic or exothermic as written? That matters! • Endothermic: Heats acts like a reactant; adding heat drives a reaction toward products. • Exothermic: Heat acts like a product; adding heat drives a reaction toward reactants. Equilibrium © 2015 Pearson Education, Inc.
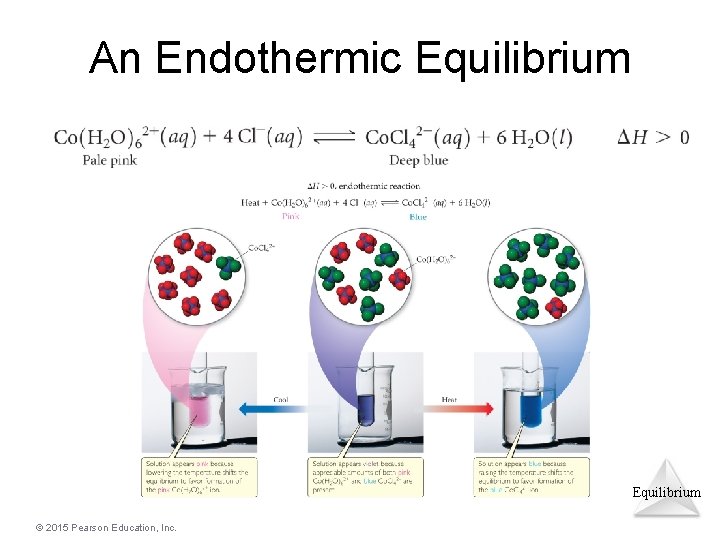
An Endothermic Equilibrium © 2015 Pearson Education, Inc.

An Exothermic Equilibrium • The Haber Process for producing ammonia from the elements is exothermic. • One would think that cooling down the reactants would result in more product. • However, the activation energy for this reaction is high! • This is the one instance where a system in equilibrium can be affected by a catalyst! Equilibrium © 2015 Pearson Education, Inc.
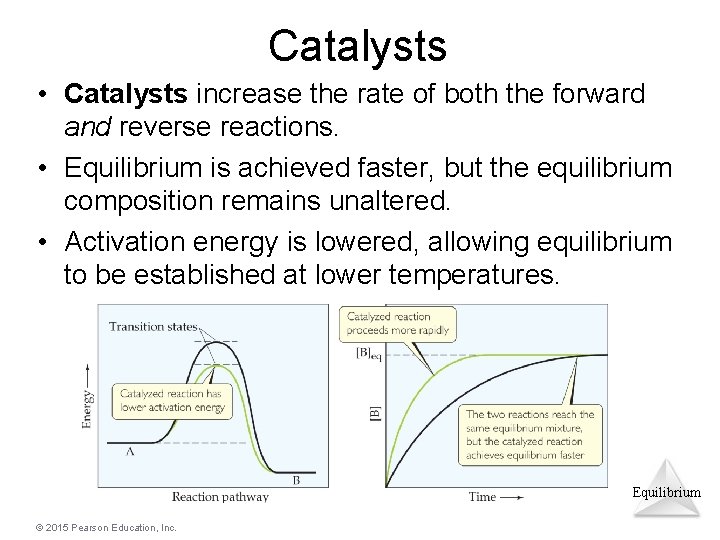
Catalysts • Catalysts increase the rate of both the forward and reverse reactions. • Equilibrium is achieved faster, but the equilibrium composition remains unaltered. • Activation energy is lowered, allowing equilibrium to be established at lower temperatures. Equilibrium © 2015 Pearson Education, Inc.
- Slides: 21