Lecture Presentation Chapter 5 The Atomic Nucleus Bradley

Lecture Presentation Chapter 5 The Atomic Nucleus Bradley Sieve Northern Kentucky University Highland Heights, KY © 2014 Pearson Education, Inc.
5. 1 Radioactivity Results from Unstable Nuclei • Stable Nuclei – Due to the ratio of protons to neutrons • Unstable Nuclei – Contains an “off-balance” ratio of protons and neutrons – Transforms the nucleus to a more stable composition © 2014 Pearson Education, Inc.

5. 1 Radioactivity Results from Unstable Nuclei • Radioactive Materials – Material containing unstable nuclei • Radioactivity – Emitted high-energy particles and radiation from unstable nuclei • Radioactive Decay – Process of emitting radioactivity © 2014 Pearson Education, Inc.

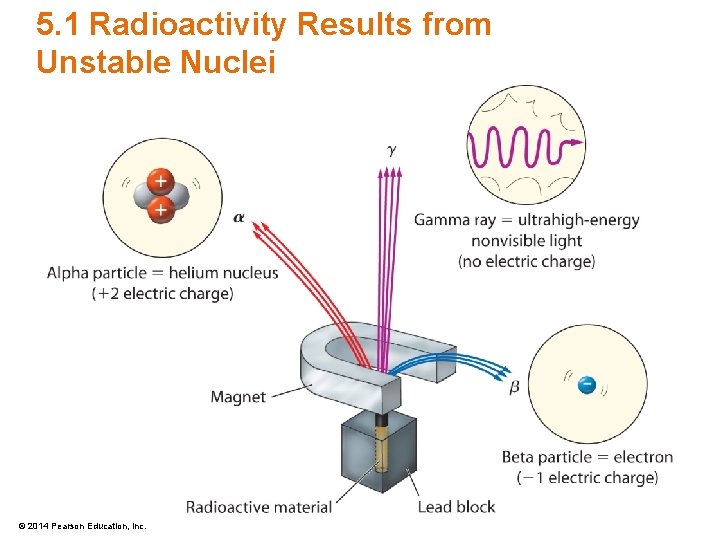
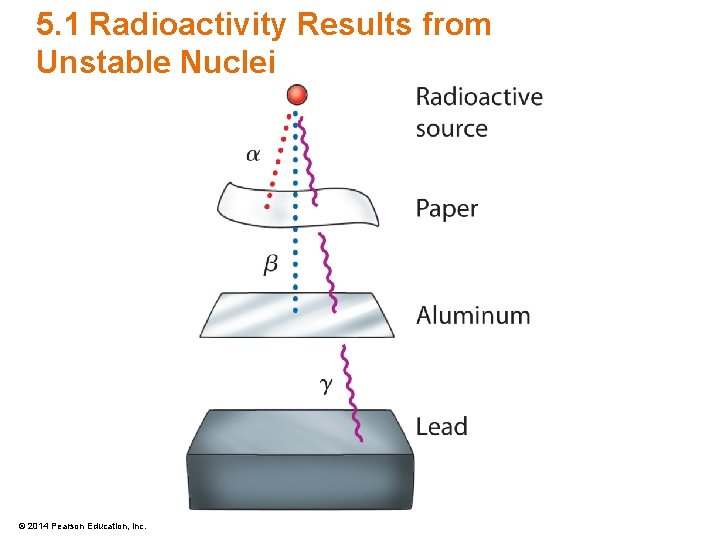
5. 1 Radioactivity Results from Unstable Nuclei • Three types of radiation are emitted – Alpha (α) • Alpha particles carry a positive electric charge – Beta (β) • Beta particles carry a negative electric charge – Gamma (γ) • Gamma particles carry no electric charge © 2014 Pearson Education, Inc.
5. 1 Radioactivity Results from Unstable Nuclei © 2014 Pearson Education, Inc.
5. 1 Radioactivity Results from Unstable Nuclei • Alpha Radiation – Releases a stream of alpha particles • Alpha Particle – Contains two protons and two neutrons – The same as a helium nuclei – Low penetrating power due to large mass (2 a. m. u. ) and double positive charge (+2) © 2014 Pearson Education, Inc.
5. 1 Radioactivity Results from Unstable Nuclei • Beta Radiation – Releases a stream of beta particles • Beta Particle – Is simply an electron ejected – Medium-range penetrating power with small mass and a single negative charge © 2014 Pearson Education, Inc.
5. 1 Radioactivity Results from Unstable Nuclei • Gamma Radiation – High-frequency electromagnetic radiation • Gamma Particle – Is pure energy – Highest penetrating power as there is no mass or charge with the particle © 2014 Pearson Education, Inc.
5. 1 Radioactivity Results from Unstable Nuclei © 2014 Pearson Education, Inc.

5. 1 Radioactivity Results from Unstable Nuclei © 2014 Pearson Education, Inc.
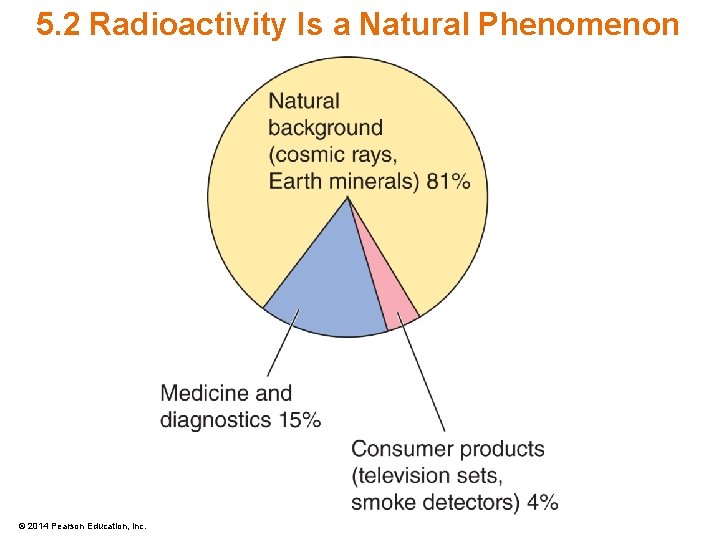
5. 2 Radioactivity Is a Natural Phenomenon • Radioactivity has always been present – Contained in soil, air, the earth’s core • Most radiation is natural background radiation – 81% natural sources – 15% medical and diagnostic sources – 4% consumer products © 2014 Pearson Education, Inc.
5. 2 Radioactivity Is a Natural Phenomenon © 2014 Pearson Education, Inc.

5. 2 Radioactivity Is a Natural Phenomenon • Radon-222 – Is a common source of radiation – Arises from uranium rocks – Can collect in basements to unsafe levels © 2014 Pearson Education, Inc.
5. 2 Radioactivity Is a Natural Phenomenon • Mutations – Alterations in genetic information contained in our cells – Normally harmless but may cause conditions such as many types of cancer – May be passed on to offspring if damage is in a person’s reproductive cells © 2014 Pearson Education, Inc.
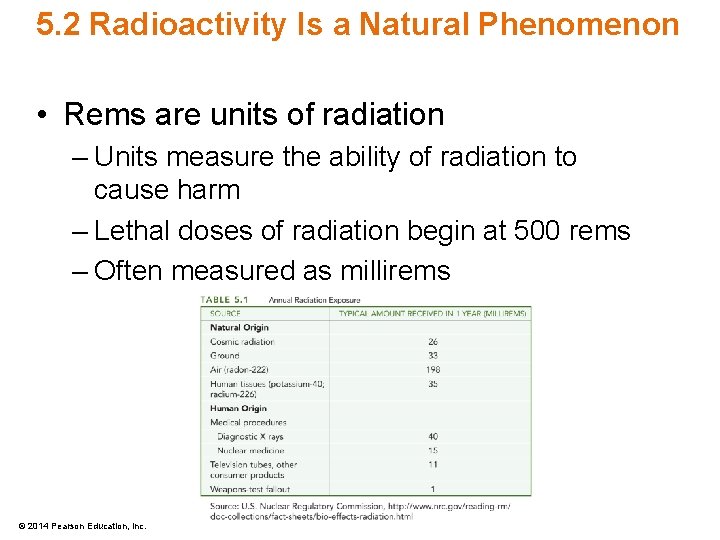
5. 2 Radioactivity Is a Natural Phenomenon • Rems are units of radiation – Units measure the ability of radiation to cause harm – Lethal doses of radiation begin at 500 rems – Often measured as millirems © 2014 Pearson Education, Inc.
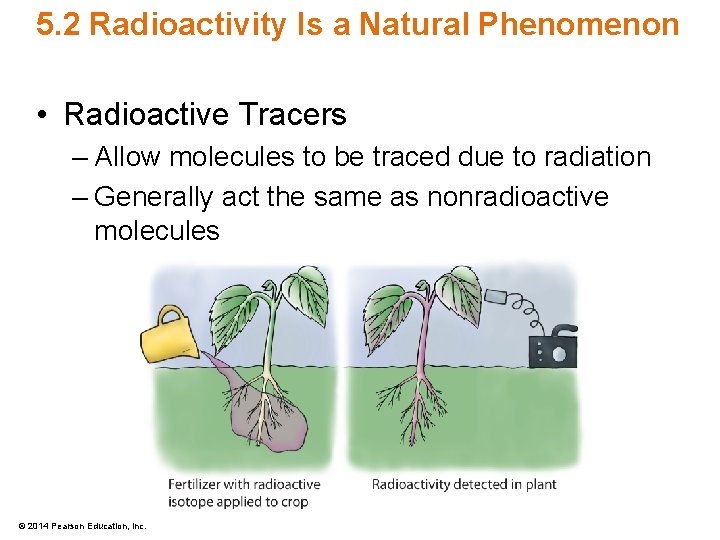
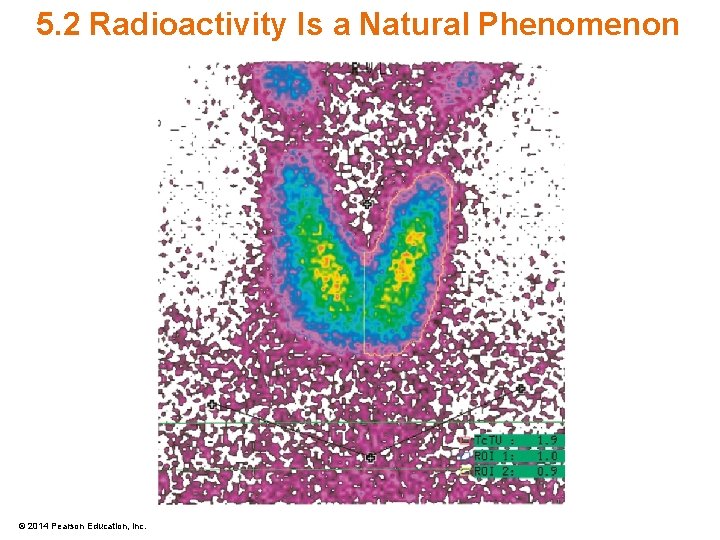
5. 2 Radioactivity Is a Natural Phenomenon • Radioactive Tracers – Allow molecules to be traced due to radiation – Generally act the same as nonradioactive molecules © 2014 Pearson Education, Inc.
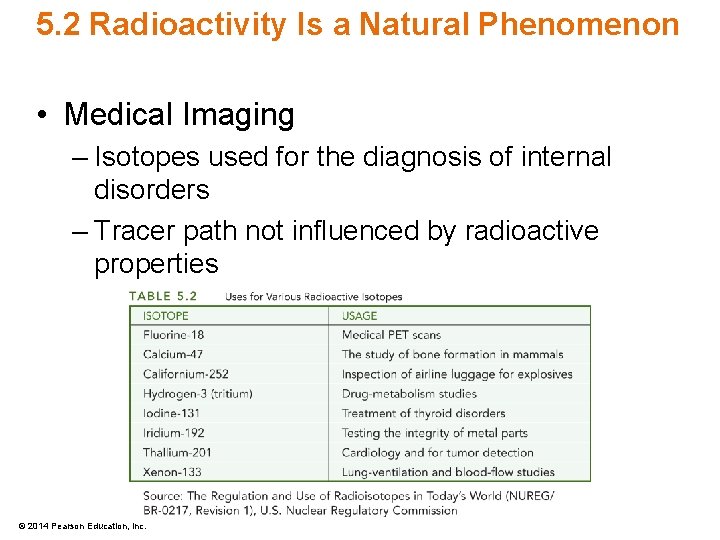
5. 2 Radioactivity Is a Natural Phenomenon • Medical Imaging – Isotopes used for the diagnosis of internal disorders – Tracer path not influenced by radioactive properties © 2014 Pearson Education, Inc.
5. 2 Radioactivity Is a Natural Phenomenon © 2014 Pearson Education, Inc.
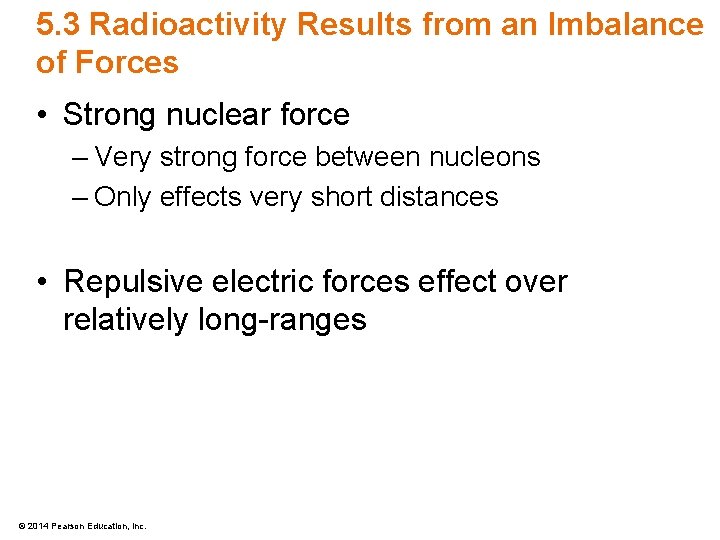
5. 3 Radioactivity Results from an Imbalance of Forces • Strong nuclear force – Very strong force between nucleons – Only effects very short distances • Repulsive electric forces effect over relatively long-ranges © 2014 Pearson Education, Inc.
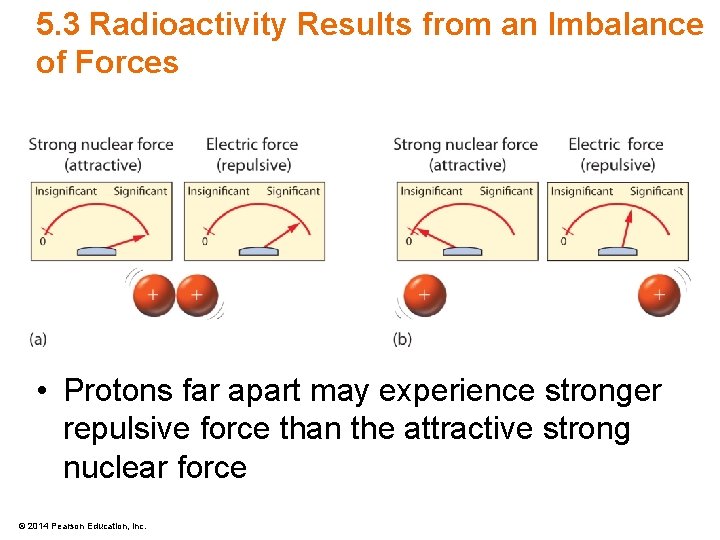
5. 3 Radioactivity Results from an Imbalance of Forces • Protons far apart may experience stronger repulsive force than the attractive strong nuclear force © 2014 Pearson Education, Inc.
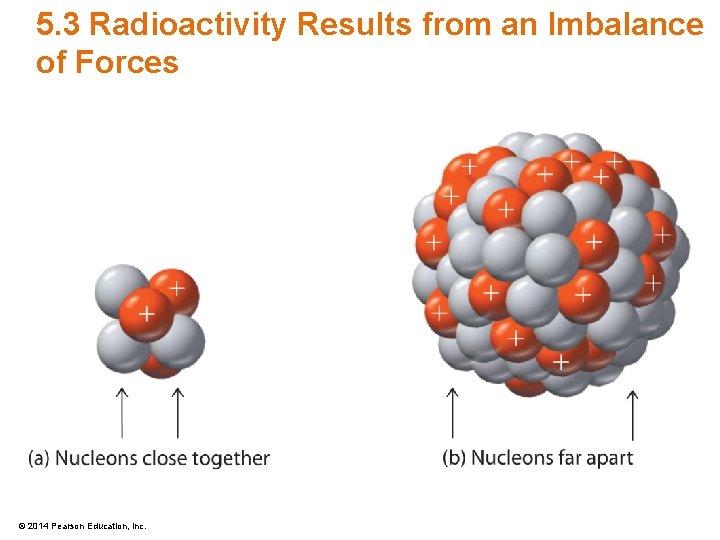
5. 3 Radioactivity Results from an Imbalance of Forces © 2014 Pearson Education, Inc.

Concept Check Two protons in the atomic nucleus repel each other, but they are also attracted to each other. Why? © 2014 Pearson Education, Inc.
Concept Check While two protons repel each other by the electric force, they also attract each other by the strong nuclear force. These forces act simultaneously. © 2014 Pearson Education, Inc.
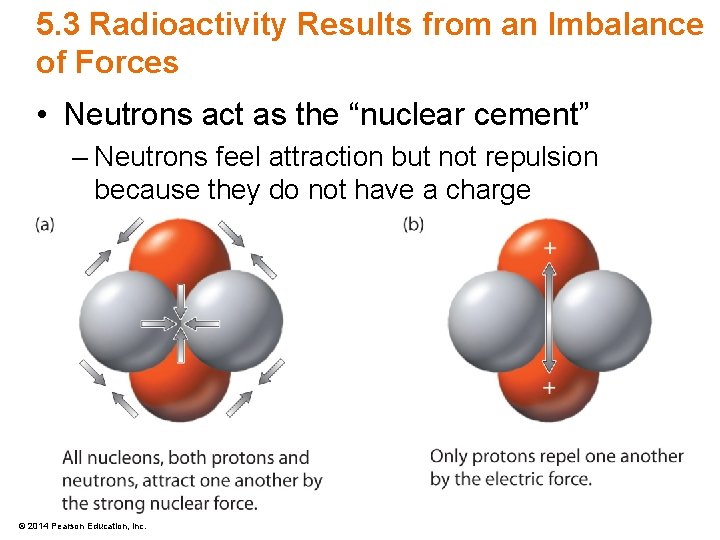
5. 3 Radioactivity Results from an Imbalance of Forces • Neutrons act as the “nuclear cement” – Neutrons feel attraction but not repulsion because they do not have a charge © 2014 Pearson Education, Inc.
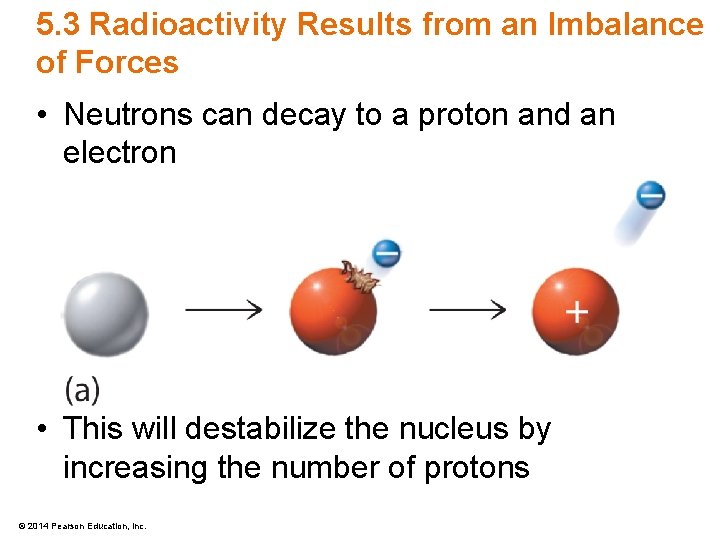
5. 3 Radioactivity Results from an Imbalance of Forces • Neutrons can decay to a proton and an electron • This will destabilize the nucleus by increasing the number of protons © 2014 Pearson Education, Inc.
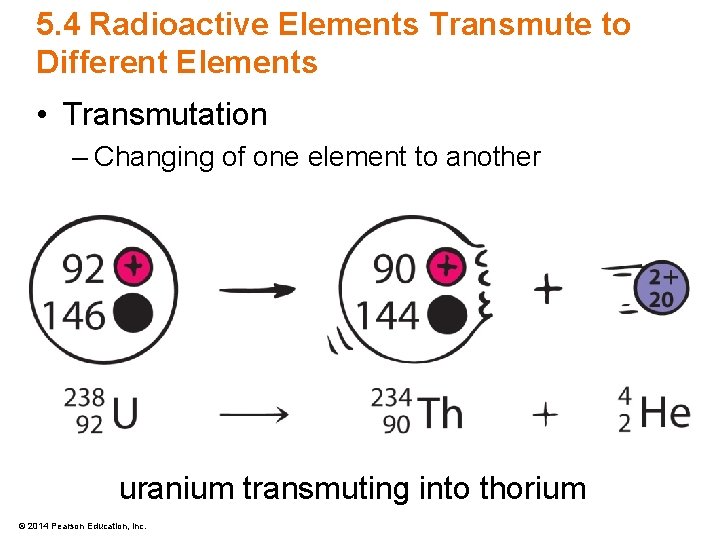
5. 4 Radioactive Elements Transmute to Different Elements • Transmutation – Changing of one element to another uranium transmuting into thorium © 2014 Pearson Education, Inc.
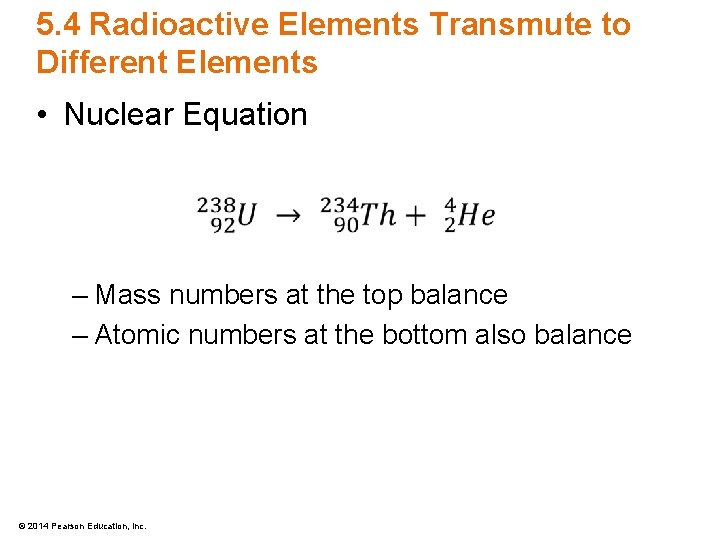
5. 4 Radioactive Elements Transmute to Different Elements • Nuclear Equation – Mass numbers at the top balance – Atomic numbers at the bottom also balance © 2014 Pearson Education, Inc.
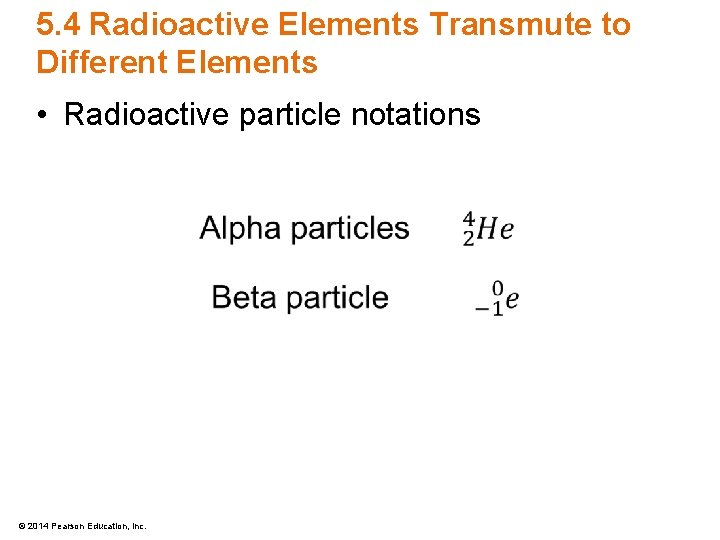
5. 4 Radioactive Elements Transmute to Different Elements • Radioactive particle notations © 2014 Pearson Education, Inc.
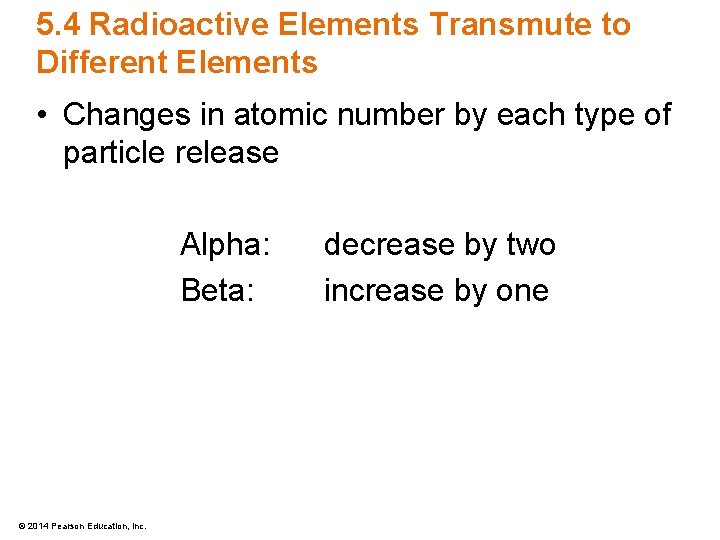
5. 4 Radioactive Elements Transmute to Different Elements • Changes in atomic number by each type of particle release Alpha: Beta: © 2014 Pearson Education, Inc. decrease by two increase by one
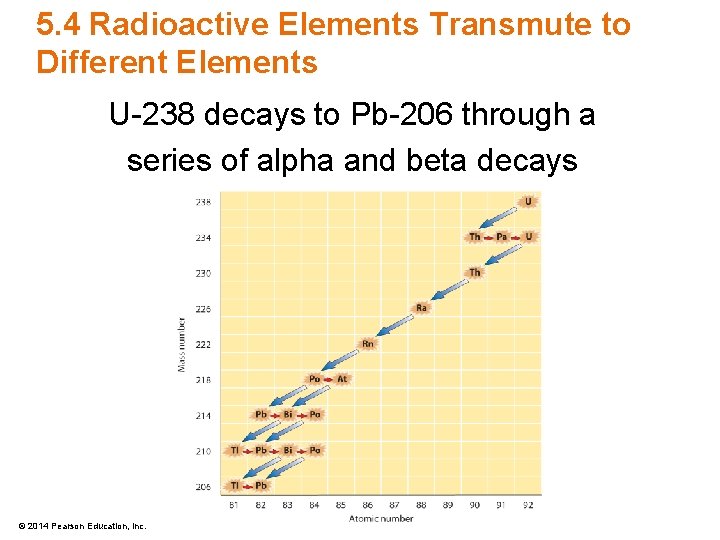
5. 4 Radioactive Elements Transmute to Different Elements U-238 decays to Pb-206 through a series of alpha and beta decays © 2014 Pearson Education, Inc.
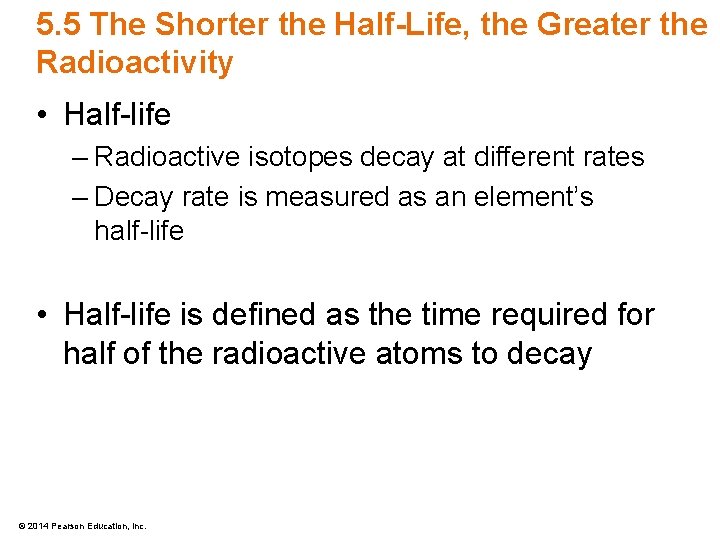
5. 5 The Shorter the Half-Life, the Greater the Radioactivity • Half-life – Radioactive isotopes decay at different rates – Decay rate is measured as an element’s half-life • Half-life is defined as the time required for half of the radioactive atoms to decay © 2014 Pearson Education, Inc.
5. 5 The Shorter the Half-Life, the Greater the Radioactivity © 2014 Pearson Education, Inc.
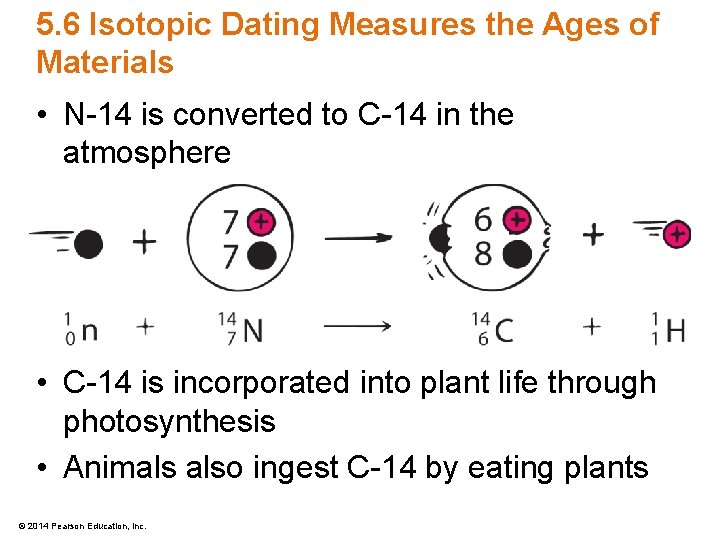
5. 6 Isotopic Dating Measures the Ages of Materials • N-14 is converted to C-14 in the atmosphere • C-14 is incorporated into plant life through photosynthesis • Animals also ingest C-14 by eating plants © 2014 Pearson Education, Inc.
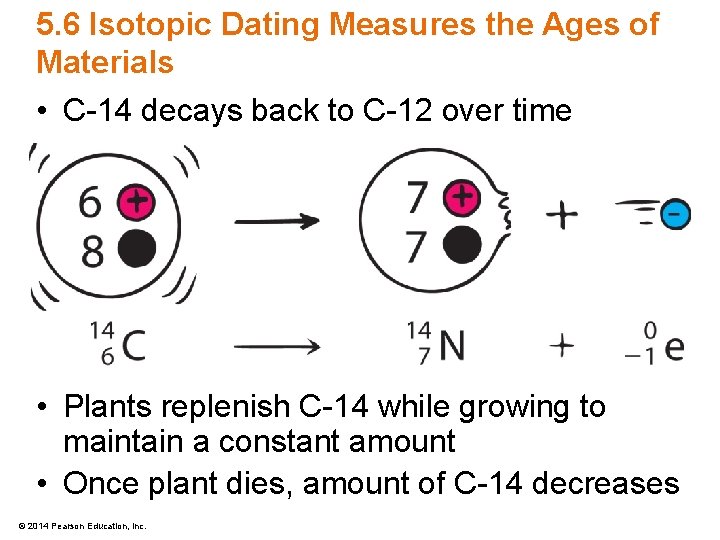
5. 6 Isotopic Dating Measures the Ages of Materials • C-14 decays back to C-12 over time • Plants replenish C-14 while growing to maintain a constant amount • Once plant dies, amount of C-14 decreases © 2014 Pearson Education, Inc.

5. 6 Isotopic Dating Measures the Ages of Materials • Carbon-14 Dating – Used to calculate the age of carbon-containing artifacts – Half of the C-14 decays in about 5730 years – Exhibits roughly a 15% error rate © 2014 Pearson Education, Inc.
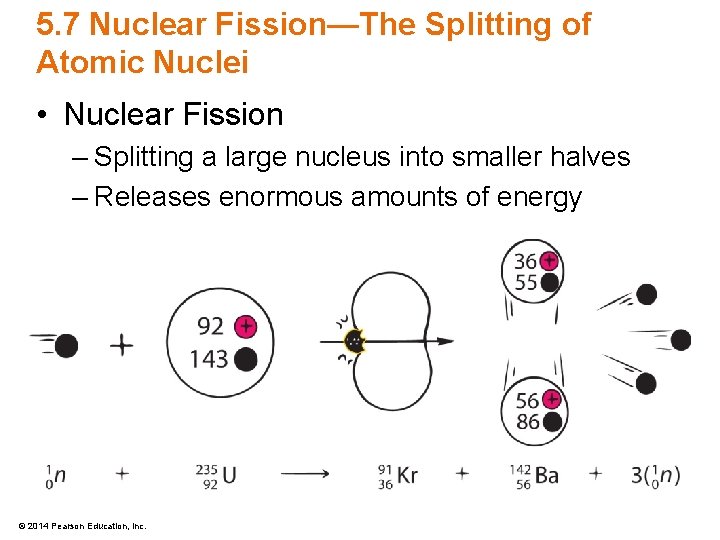
5. 7 Nuclear Fission—The Splitting of Atomic Nuclei • Nuclear Fission – Splitting a large nucleus into smaller halves – Releases enormous amounts of energy © 2014 Pearson Education, Inc.
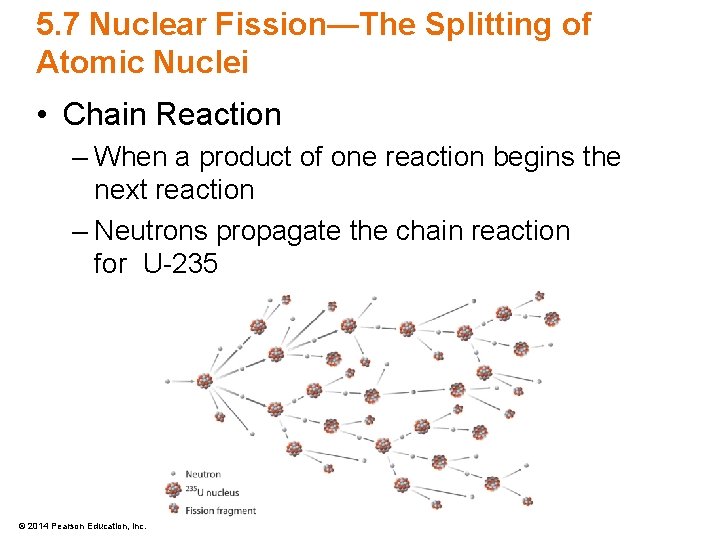
5. 7 Nuclear Fission—The Splitting of Atomic Nuclei • Chain Reaction – When a product of one reaction begins the next reaction – Neutrons propagate the chain reaction for U-235 © 2014 Pearson Education, Inc.
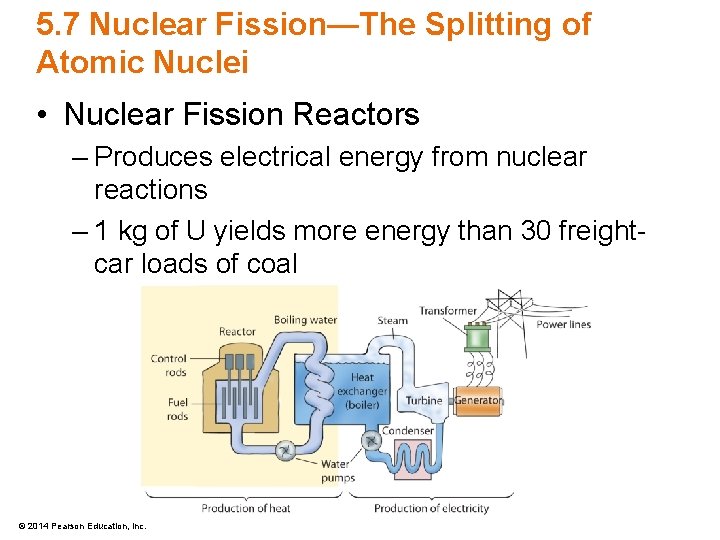
5. 7 Nuclear Fission—The Splitting of Atomic Nuclei • Nuclear Fission Reactors – Produces electrical energy from nuclear reactions – 1 kg of U yields more energy than 30 freightcar loads of coal © 2014 Pearson Education, Inc.
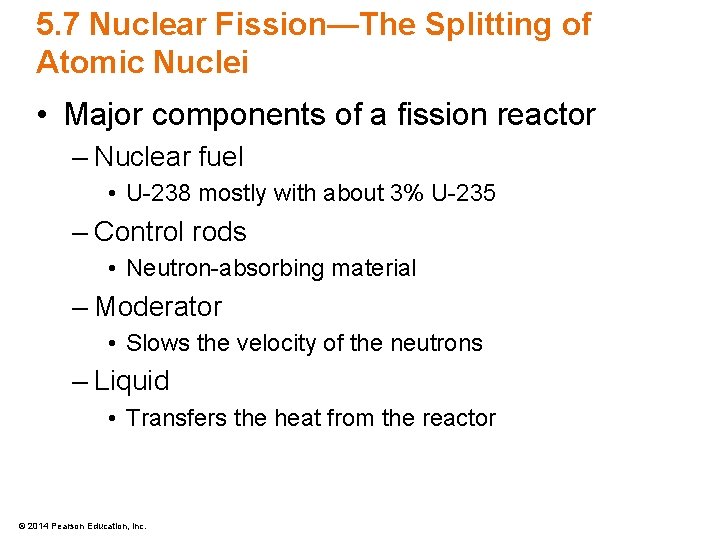
5. 7 Nuclear Fission—The Splitting of Atomic Nuclei • Major components of a fission reactor – Nuclear fuel • U-238 mostly with about 3% U-235 – Control rods • Neutron-absorbing material – Moderator • Slows the velocity of the neutrons – Liquid • Transfers the heat from the reactor © 2014 Pearson Education, Inc.

5. 7 Nuclear Fission—The Splitting of Atomic Nuclei • Radioactive Waste – By-products of nuclear reactions – Half-lives range from short to thousands of years – Disposal of waste is problematic © 2014 Pearson Education, Inc.
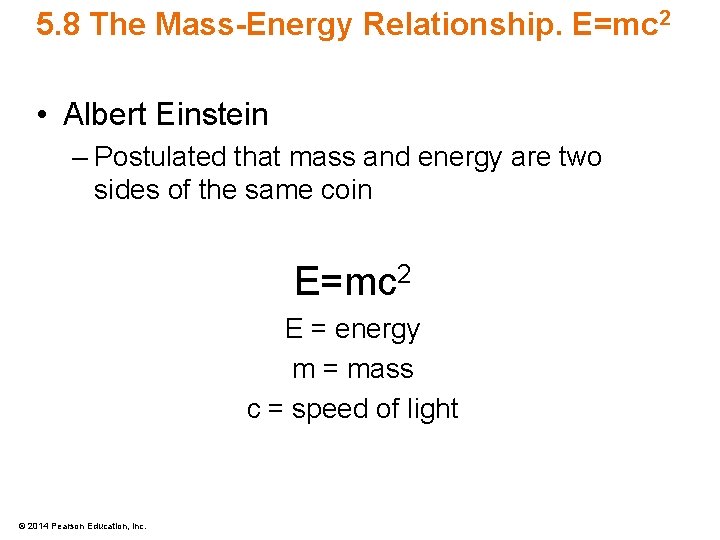
5. 8 The Mass-Energy Relationship. E=mc 2 • Albert Einstein – Postulated that mass and energy are two sides of the same coin E=mc 2 E = energy m = mass c = speed of light © 2014 Pearson Education, Inc.
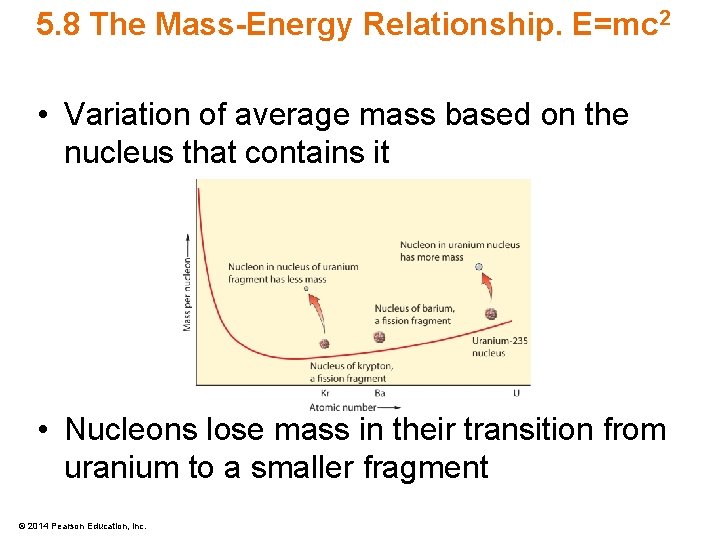
5. 8 The Mass-Energy Relationship. E=mc 2 • Variation of average mass based on the nucleus that contains it • Nucleons lose mass in their transition from uranium to a smaller fragment © 2014 Pearson Education, Inc.
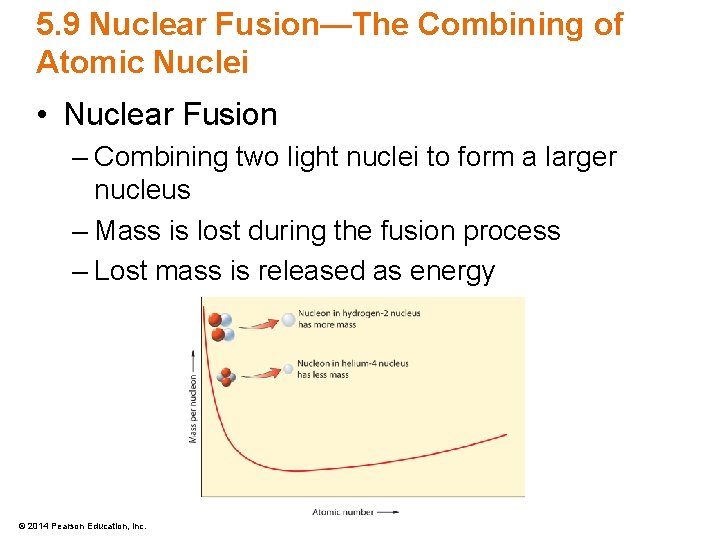
5. 9 Nuclear Fusion—The Combining of Atomic Nuclei • Nuclear Fusion – Combining two light nuclei to form a larger nucleus – Mass is lost during the fusion process – Lost mass is released as energy © 2014 Pearson Education, Inc.
5. 9 Nuclear Fusion—The Combining of Atomic Nuclei • Thermonuclear Fusion – Fusion brought about by high temperatures – Can be initiated by a fission reaction © 2014 Pearson Education, Inc.

5. 9 Nuclear Fusion—The Combining of Atomic Nuclei • Controlling fusion can be done using plasmas – Magnetic straitjacket for hot ionized gases – Currently no commercial fusion power plants in use © 2014 Pearson Education, Inc.
- Slides: 45