Lecture Presentation Chapter 11 Oxidation and Reductions Charge



















- Slides: 56

Lecture Presentation Chapter 11 Oxidation and Reductions Charge the World Bradley Sieve Northern Kentucky University Highland Heights, KY © 2014 Pearson Education, Inc.

11. 1 Losing and Gaining Electrons • Oxidation – Reactant loses one or more electrons • Reduction – Reactant gains one or more electrons • Oxidation and reduction always occur together – When lost, electrons must go somewhere © 2014 Pearson Education, Inc.

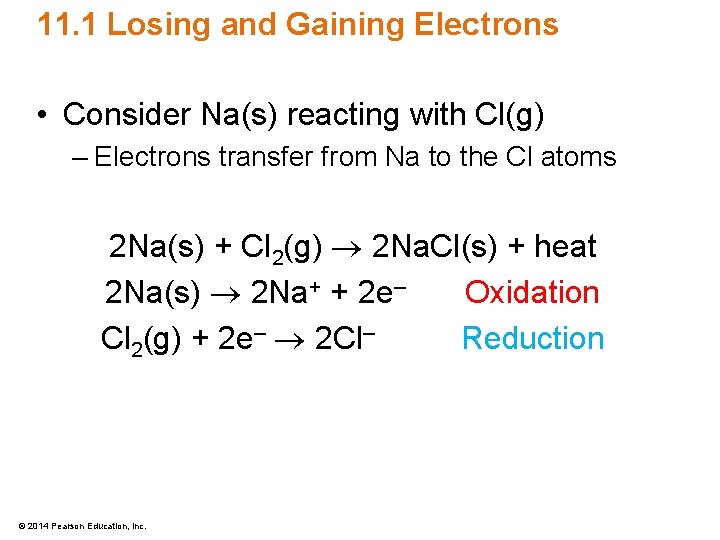
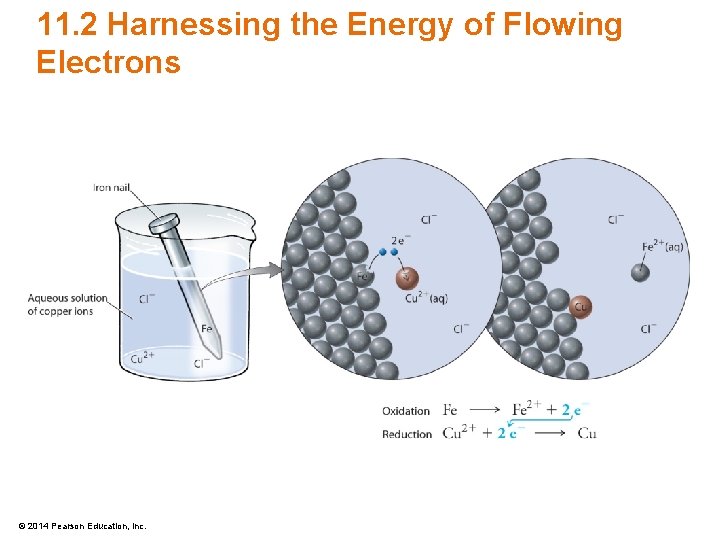
11. 1 Losing and Gaining Electrons • Consider Na(s) reacting with Cl(g) – Electrons transfer from Na to the Cl atoms 2 Na(s) + Cl 2(g) 2 Na. Cl(s) + heat 2 Na(s) 2 Na+ + 2 e– Oxidation Cl 2(g) + 2 e– 2 Cl– Reduction © 2014 Pearson Education, Inc.

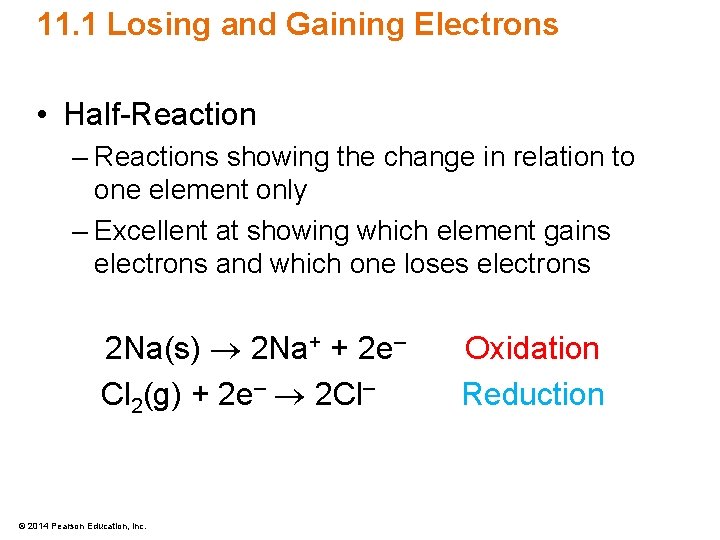
11. 1 Losing and Gaining Electrons • Half-Reaction – Reactions showing the change in relation to one element only – Excellent at showing which element gains electrons and which one loses electrons 2 Na(s) 2 Na+ + 2 e– Cl 2(g) + 2 e– 2 Cl– © 2014 Pearson Education, Inc. Oxidation Reduction

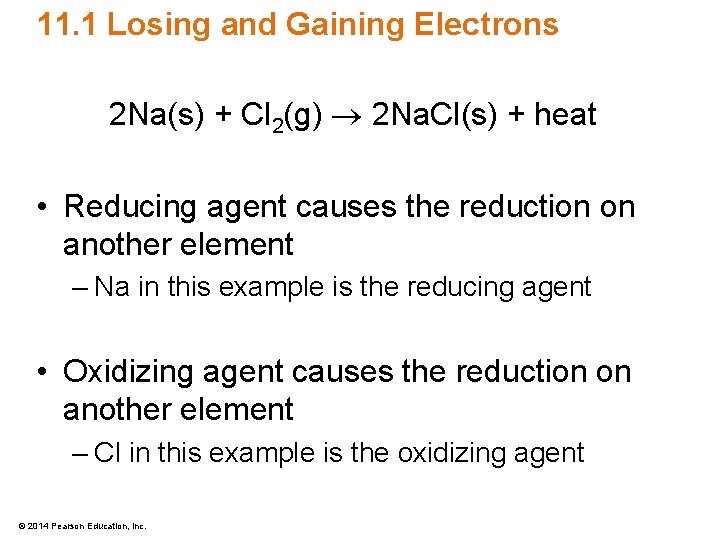
11. 1 Losing and Gaining Electrons 2 Na(s) + Cl 2(g) 2 Na. Cl(s) + heat • Reducing agent causes the reduction on another element – Na in this example is the reducing agent • Oxidizing agent causes the reduction on another element – Cl in this example is the oxidizing agent © 2014 Pearson Education, Inc.

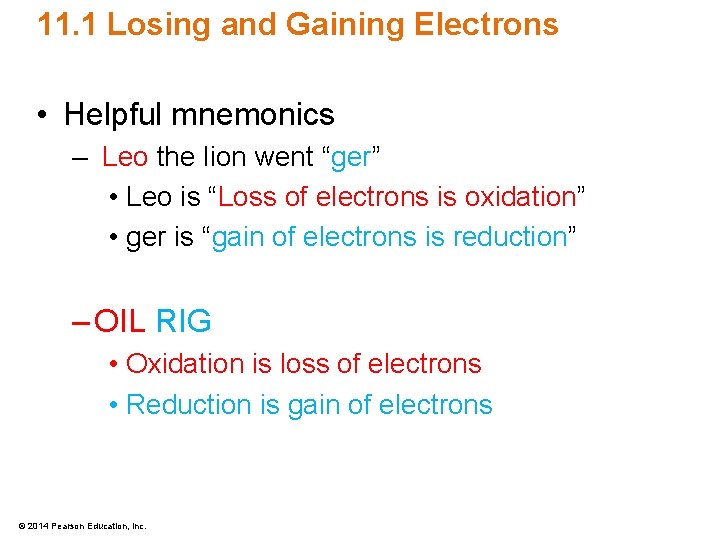
11. 1 Losing and Gaining Electrons • Helpful mnemonics – Leo the lion went “ger” • Leo is “Loss of electrons is oxidation” • ger is “gain of electrons is reduction” – OIL RIG • Oxidation is loss of electrons • Reduction is gain of electrons © 2014 Pearson Education, Inc.

Concept Check True or false: 1. Reducing agents are oxidized in oxidation -reduction reactions. 2. Oxidizing agents are reduced in oxidationreduction reactions. © 2014 Pearson Education, Inc.

Concept Check Both statements are true. © 2014 Pearson Education, Inc.

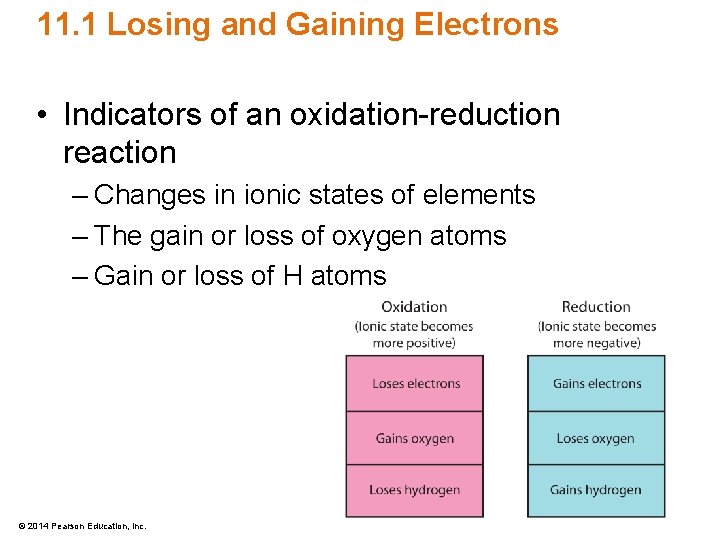
11. 1 Losing and Gaining Electrons • Indicators of an oxidation-reduction reaction – Changes in ionic states of elements – The gain or loss of oxygen atoms – Gain or loss of H atoms © 2014 Pearson Education, Inc.

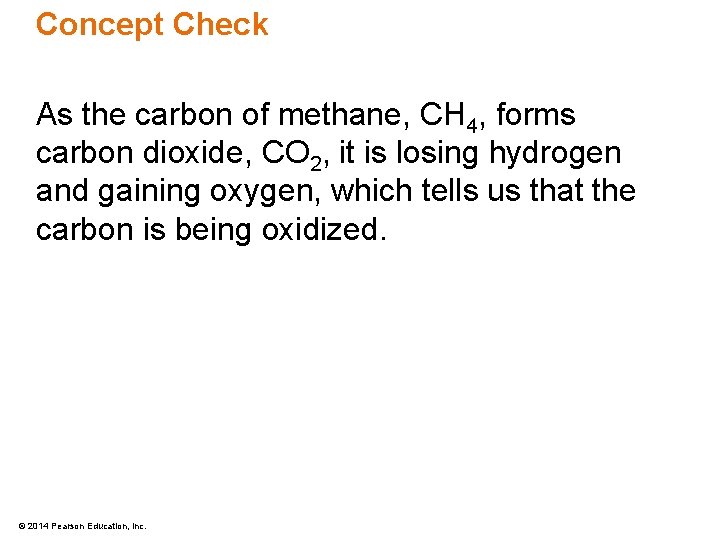
Concept Check In the following equation, is carbon oxidized or reduced? CH 4 + 2 O 2 CO 2 + 2 H 2 O © 2014 Pearson Education, Inc.

Concept Check As the carbon of methane, CH 4, forms carbon dioxide, CO 2, it is losing hydrogen and gaining oxygen, which tells us that the carbon is being oxidized. © 2014 Pearson Education, Inc.

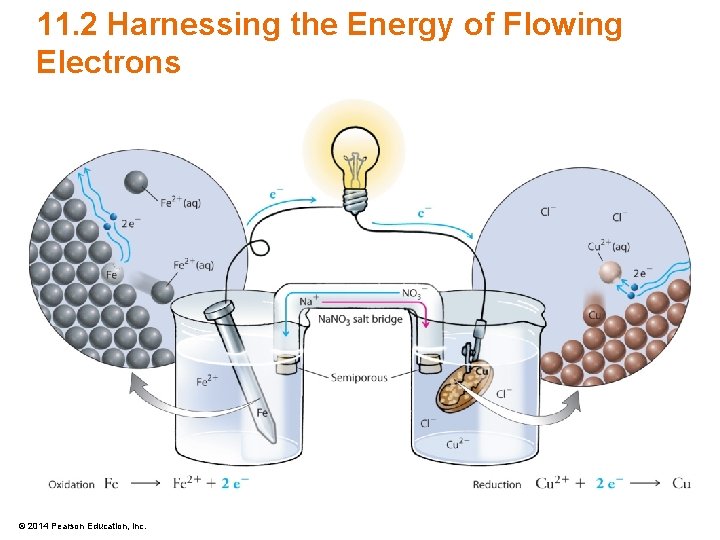
11. 2 Harnessing the Energy of Flowing Electrons • Electrochemistry – The study of the relationship between electrical energy and chemical change – Oxidation-reduction reactions can generate electricity – Flow of electrons results in electrical charges © 2014 Pearson Education, Inc.

11. 2 Harnessing the Energy of Flowing Electrons © 2014 Pearson Education, Inc.

11. 2 Harnessing the Energy of Flowing Electrons © 2014 Pearson Education, Inc.

11. 3 Batteries Consume Chemicals to Generate Electricity • Batteries – Examples of an oxidation-reduction in one container – Can be either disposable or rechargeable – Materials that oxidize and reduce each other are connected in a way that allows electron flow © 2014 Pearson Education, Inc.

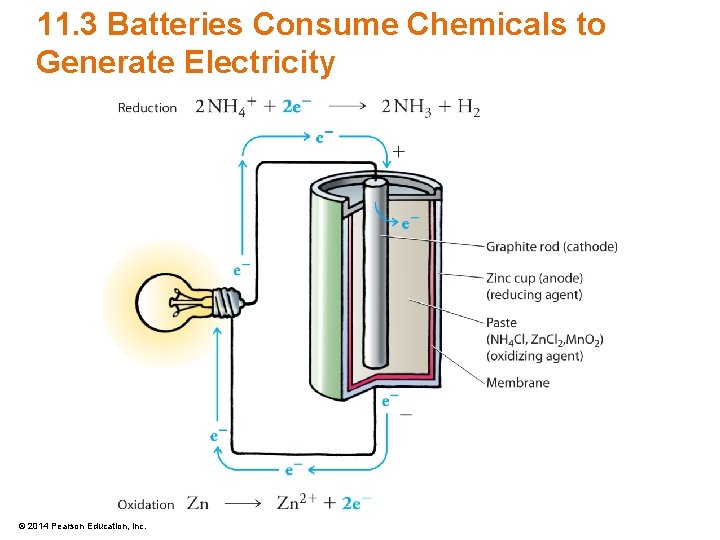
11. 3 Batteries Consume Chemicals to Generate Electricity • Dry-Cell Battery – Invented in the 1860 s – Cheapest disposable battery – Zinc container filled with NH 4 Cl, Zn. Cl 2, and Mn. O 2 – Contains a graphite rod to facilitate electron motion © 2014 Pearson Education, Inc.

11. 3 Batteries Consume Chemicals to Generate Electricity © 2014 Pearson Education, Inc.

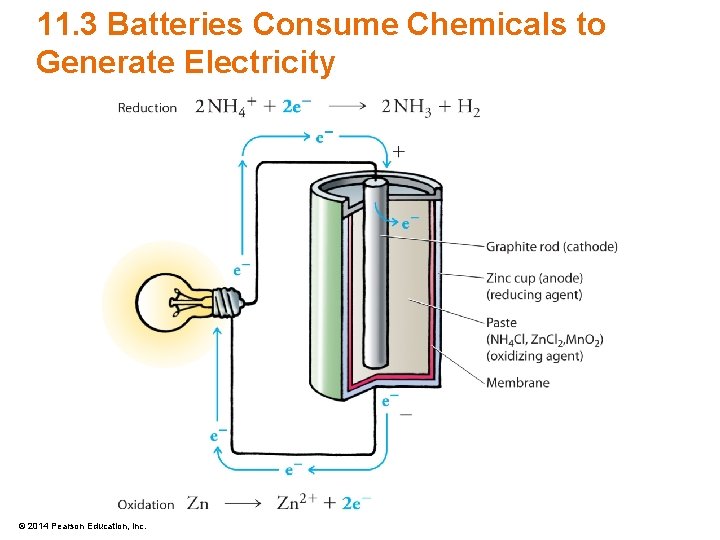
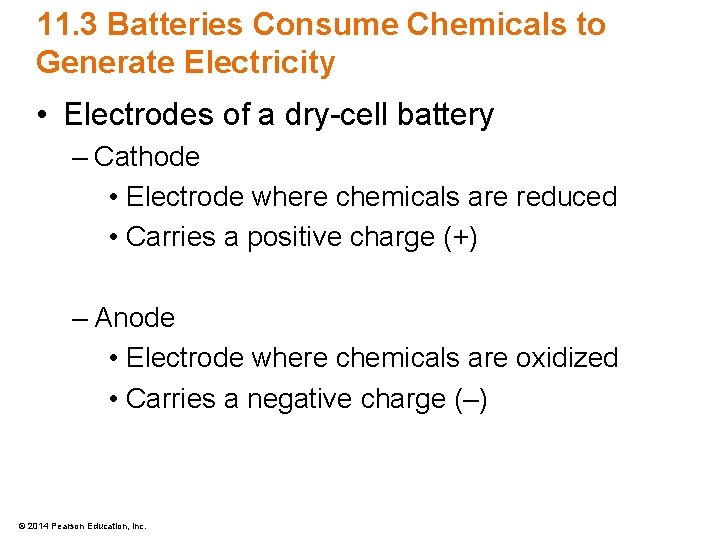
11. 3 Batteries Consume Chemicals to Generate Electricity • Electrodes of a dry-cell battery – Cathode • Electrode where chemicals are reduced • Carries a positive charge (+) – Anode • Electrode where chemicals are oxidized • Carries a negative charge (–) © 2014 Pearson Education, Inc.

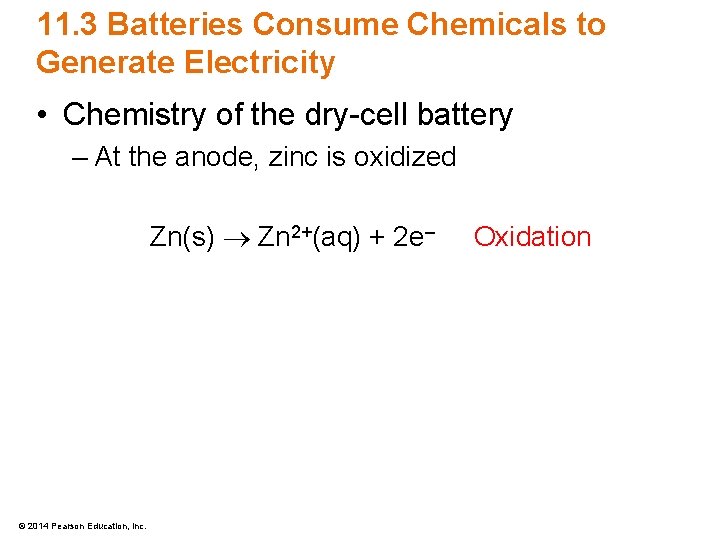
11. 3 Batteries Consume Chemicals to Generate Electricity • Chemistry of the dry-cell battery – At the anode, zinc is oxidized Zn(s) Zn 2+(aq) + 2 e– © 2014 Pearson Education, Inc. Oxidation

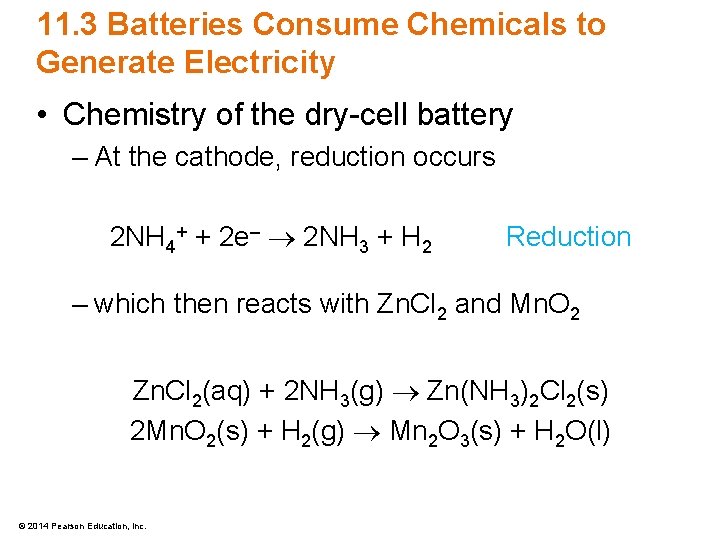
11. 3 Batteries Consume Chemicals to Generate Electricity • Chemistry of the dry-cell battery – At the cathode, reduction occurs 2 NH 4+ + 2 e– 2 NH 3 + H 2 Reduction – which then reacts with Zn. Cl 2 and Mn. O 2 Zn. Cl 2(aq) + 2 NH 3(g) Zn(NH 3)2 Cl 2(s) 2 Mn. O 2(s) + H 2(g) Mn 2 O 3(s) + H 2 O(l) © 2014 Pearson Education, Inc.

11. 3 Batteries Consume Chemicals to Generate Electricity © 2014 Pearson Education, Inc.

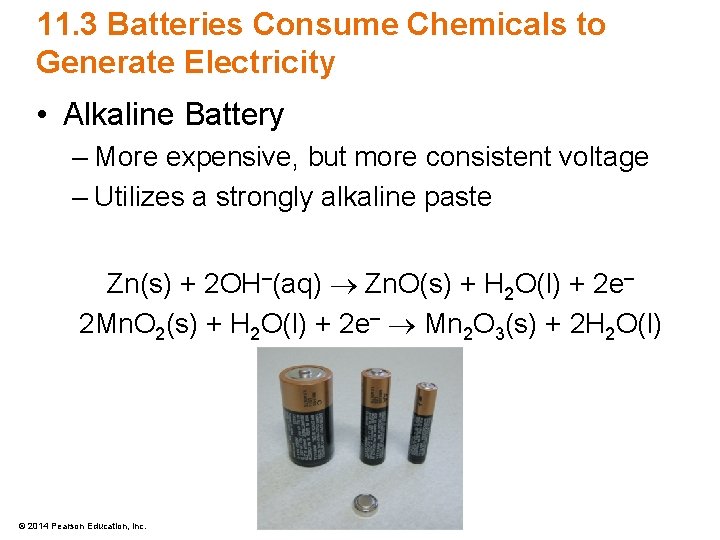
11. 3 Batteries Consume Chemicals to Generate Electricity • Alkaline Battery – More expensive, but more consistent voltage – Utilizes a strongly alkaline paste Zn(s) + 2 OH–(aq) Zn. O(s) + H 2 O(l) + 2 e– 2 Mn. O 2(s) + H 2 O(l) + 2 e– Mn 2 O 3(s) + 2 H 2 O(l) © 2014 Pearson Education, Inc.

11. 3 Batteries Consume Chemicals to Generate Electricity • Other types of disposable batteries – Mercury batteries • Use Hg. O instead of Mn. O 2 • Concerns about environmental hazards – Lithium batteries • Lithium is electron’s source instead of zinc • Maintains higher voltage and makes lighter batteries © 2014 Pearson Education, Inc.

11. 3 Batteries Consume Chemicals to Generate Electricity • Rechargeable Batteries – Contain a reversible set of oxidation and reduction reactions – One example is Ni. MH • Utilizes nickel metal and water reactants • Produces nickel hydride and hydroxide ion – Traditional car batteries are another type utilizing lead compounds © 2014 Pearson Education, Inc.

11. 3 Batteries Consume Chemicals to Generate Electricity • Rechargeable lithium-ion batteries – Widespread use, including computer laptops and cell phones – Hybrid cars use lithium phosphate batteries © 2014 Pearson Education, Inc.

Concept Check What chemicals are produced as a nickelmetal hydride battery is recharged? © 2014 Pearson Education, Inc.

Concept Check The chemicals are nickel hydride, H: Ni, and hydroxide ions, OH−. © 2014 Pearson Education, Inc.

11. 4 Fuel Cells Consume Fuel to Generate Electricity • Fuel Cell – Device that converts energy of fuel to electrical energy – Consumes a continuous supply of fuel to produce electricity – One common type is the hydrogen–oxygen fuel cell © 2014 Pearson Education, Inc.

11. 4 Fuel Cells Consume Fuel to Generate Electricity © 2014 Pearson Education, Inc.

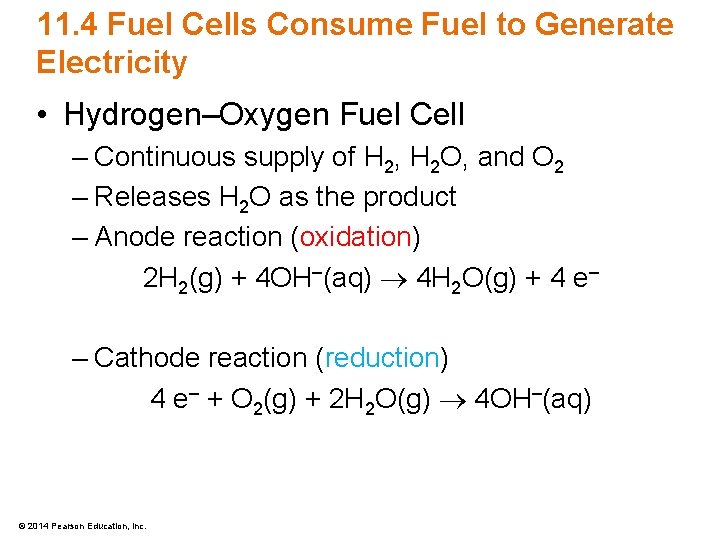
11. 4 Fuel Cells Consume Fuel to Generate Electricity • Hydrogen–Oxygen Fuel Cell – Continuous supply of H 2, H 2 O, and O 2 – Releases H 2 O as the product – Anode reaction (oxidation) 2 H 2(g) + 4 OH–(aq) 4 H 2 O(g) + 4 e– – Cathode reaction (reduction) 4 e– + O 2(g) + 2 H 2 O(g) 4 OH–(aq) © 2014 Pearson Education, Inc.

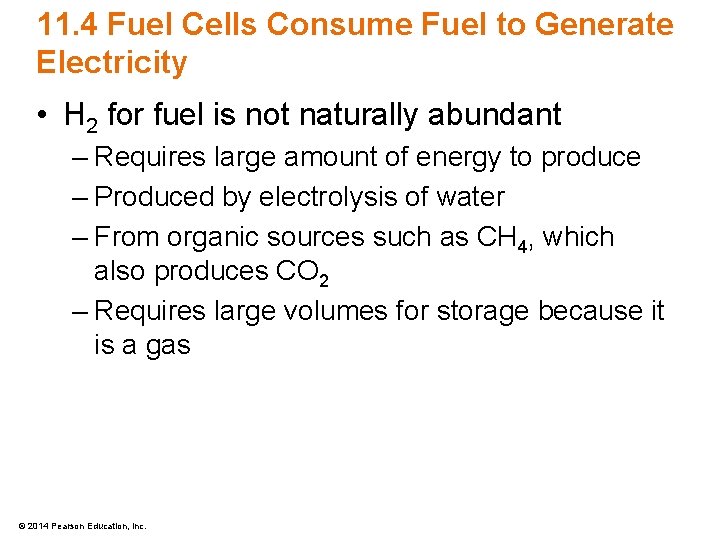
11. 4 Fuel Cells Consume Fuel to Generate Electricity • H 2 for fuel is not naturally abundant – Requires large amount of energy to produce – Produced by electrolysis of water – From organic sources such as CH 4, which also produces CO 2 – Requires large volumes for storage because it is a gas © 2014 Pearson Education, Inc.

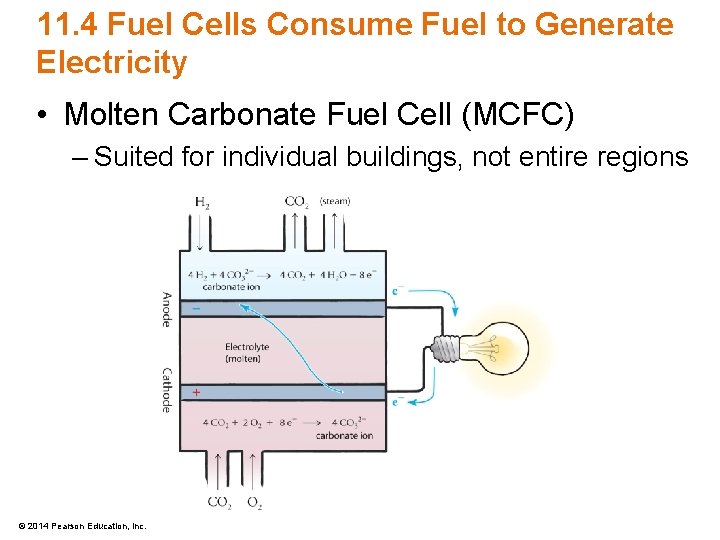
11. 4 Fuel Cells Consume Fuel to Generate Electricity • Molten Carbonate Fuel Cell (MCFC) – Suited for individual buildings, not entire regions © 2014 Pearson Education, Inc.

11. 5 Photovoltaic Transform Light into Energy • Photovoltaic Cells – Most direct means of converting sunlight to electrical energy – Used on satellites in the 1960 s – Can be installed on homes and at many other locations © 2014 Pearson Education, Inc.

11. 5 Photovoltaic Transform Light into Energy © 2014 Pearson Education, Inc.

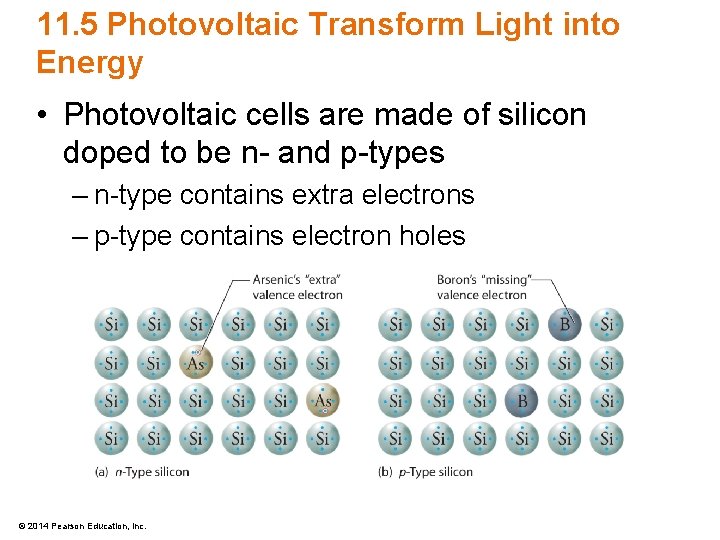
11. 5 Photovoltaic Transform Light into Energy • Photovoltaic cells are made of silicon doped to be n- and p-types – n-type contains extra electrons – p-type contains electron holes © 2014 Pearson Education, Inc.

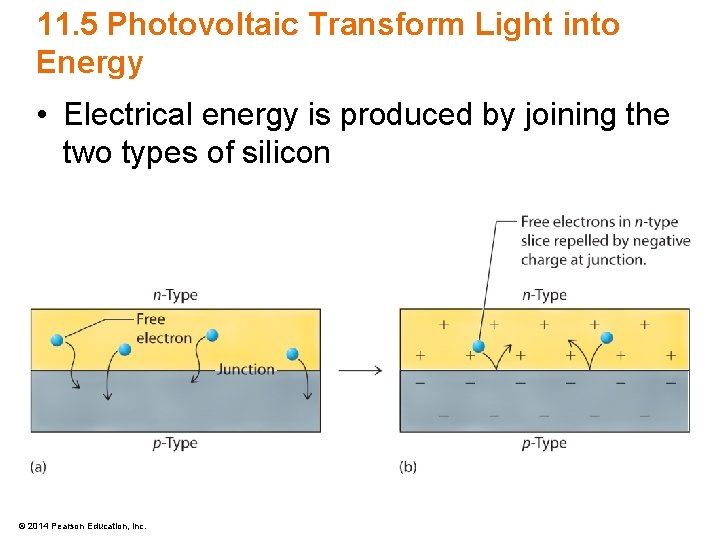
11. 5 Photovoltaic Transform Light into Energy • Electrical energy is produced by joining the two types of silicon © 2014 Pearson Education, Inc.

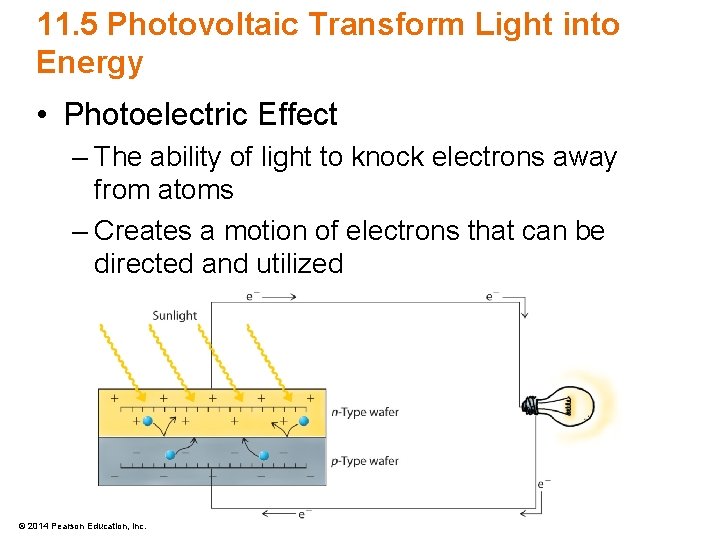
11. 5 Photovoltaic Transform Light into Energy • Photoelectric Effect – The ability of light to knock electrons away from atoms – Creates a motion of electrons that can be directed and utilized © 2014 Pearson Education, Inc.

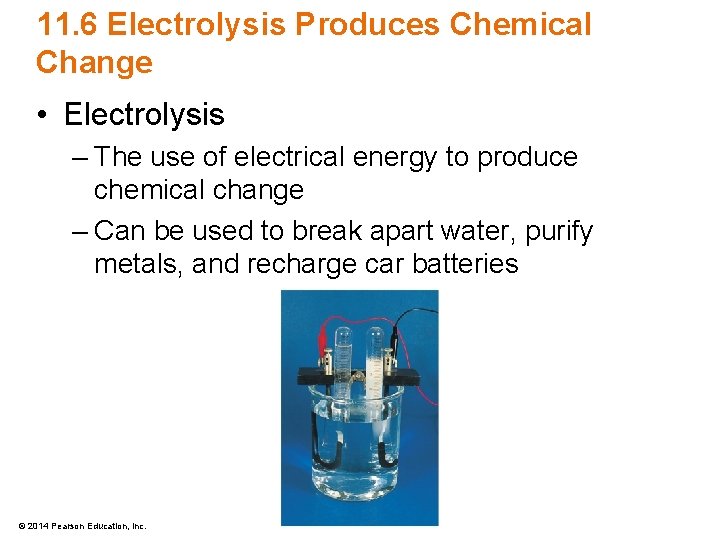
11. 6 Electrolysis Produces Chemical Change • Electrolysis – The use of electrical energy to produce chemical change – Can be used to break apart water, purify metals, and recharge car batteries © 2014 Pearson Education, Inc.

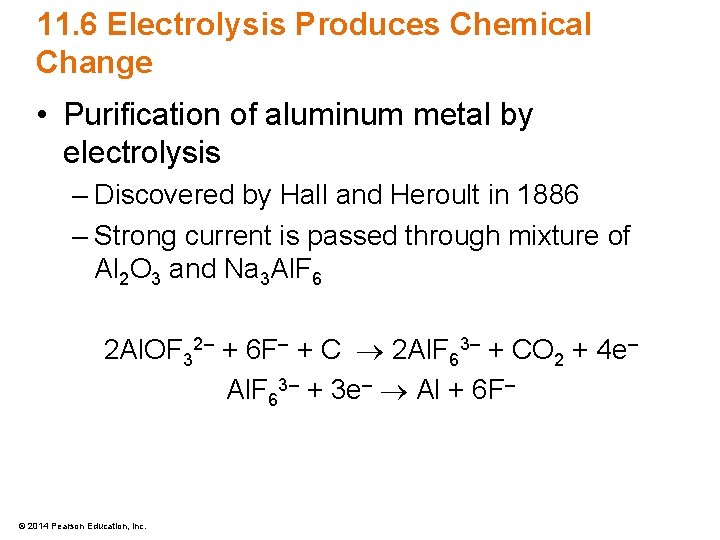
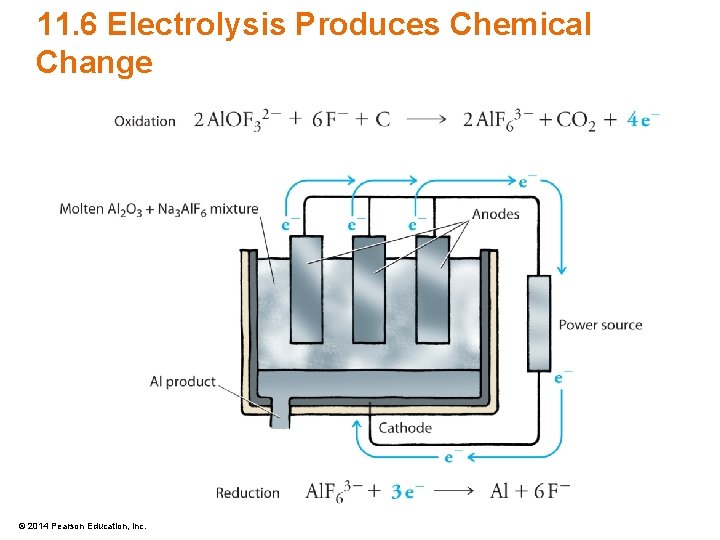
11. 6 Electrolysis Produces Chemical Change • Purification of aluminum metal by electrolysis – Discovered by Hall and Heroult in 1886 – Strong current is passed through mixture of Al 2 O 3 and Na 3 Al. F 6 2 Al. OF 32– + 6 F– + C 2 Al. F 63– + CO 2 + 4 e– Al. F 63– + 3 e– Al + 6 F– © 2014 Pearson Education, Inc.

11. 6 Electrolysis Produces Chemical Change © 2014 Pearson Education, Inc.

Concept Check Is the exothermic reaction in a hydrogen– oxygen fuel cell an example of electrolysis? © 2014 Pearson Education, Inc.

Concept Check No. During electrolysis, electrical energy is used to produce chemical change. In the hydrogen–oxygen fuel cell, chemical change is used to produce electrical energy. © 2014 Pearson Education, Inc.

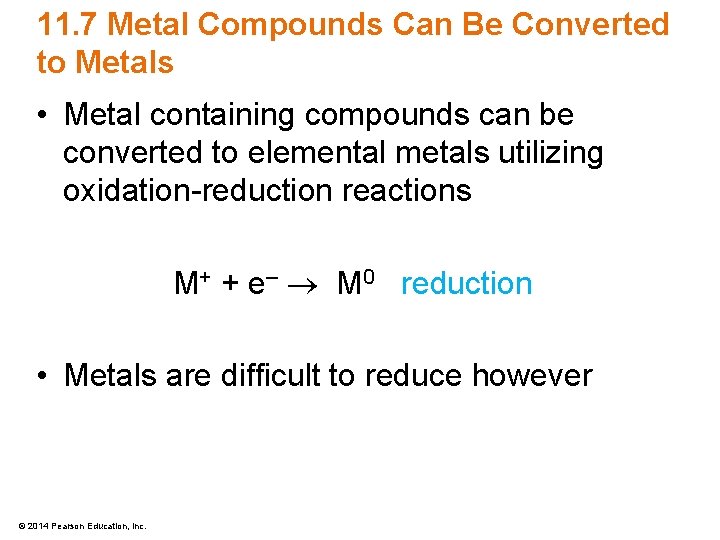
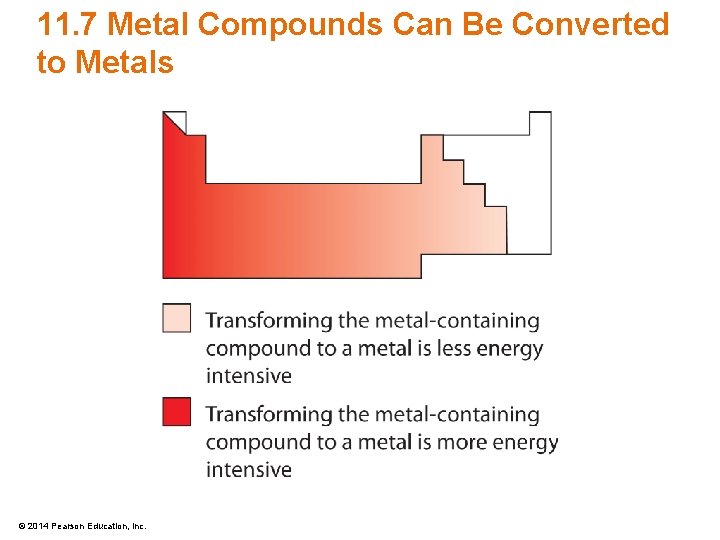
11. 7 Metal Compounds Can Be Converted to Metals • Metal containing compounds can be converted to elemental metals utilizing oxidation-reduction reactions M+ + e– M 0 reduction • Metals are difficult to reduce however © 2014 Pearson Education, Inc.

11. 7 Metal Compounds Can Be Converted to Metals © 2014 Pearson Education, Inc.

Concept Check Why is it so difficult to obtain a group 1 metal from a compound containing ions of that metal? © 2014 Pearson Education, Inc.

Concept Check The metal ions do not readily accept electrons to form metal atoms. © 2014 Pearson Education, Inc.

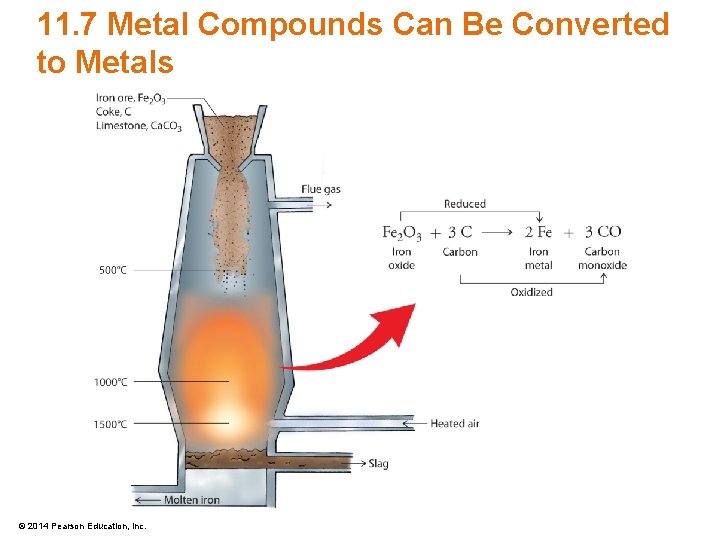
11. 7 Metal Compounds Can Be Converted to Metals • Some metals are commonly obtained from metal oxides – Blast furnaces can be used for the transformation – Electrolysis is another method © 2014 Pearson Education, Inc.

11. 7 Metal Compounds Can Be Converted to Metals © 2014 Pearson Education, Inc.

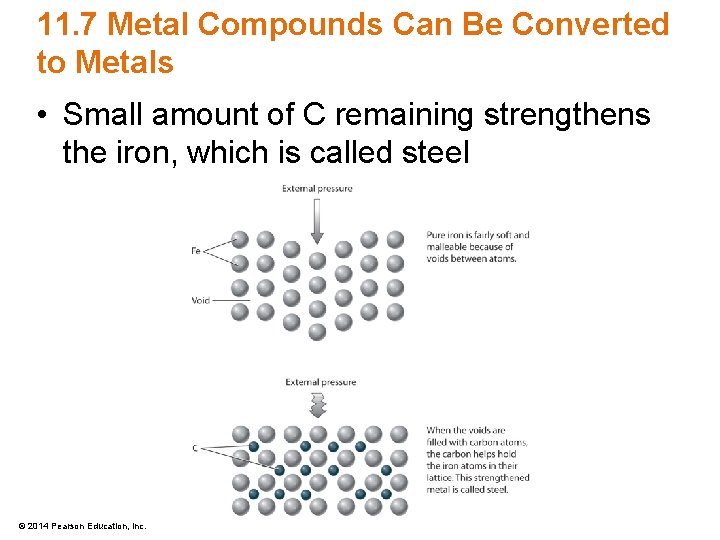
11. 7 Metal Compounds Can Be Converted to Metals • Small amount of C remaining strengthens the iron, which is called steel © 2014 Pearson Education, Inc.

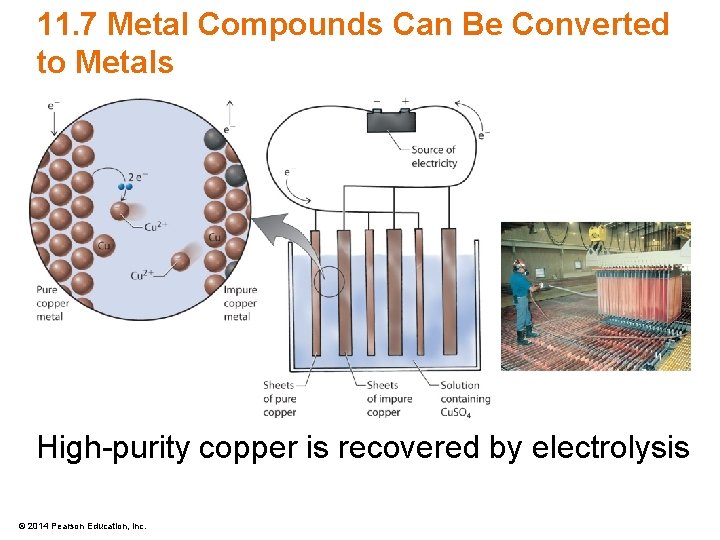
11. 7 Metal Compounds Can Be Converted to Metals High-purity copper is recovered by electrolysis © 2014 Pearson Education, Inc.

11. 7 Metal Compounds Can Be Converted to Metals • Other metals can be obtained from sulfides MS(s) + O 2 M(l) + SO 2(g) – Commonly used to purify copper © 2014 Pearson Education, Inc.

11. 8 Oxygen Is Responsible for Corrosion and Combustion • Oxygen is able to pluck electrons from other elements – Results in corrosion and combustion © 2014 Pearson Education, Inc.

11. 8 Oxygen Is Responsible for Corrosion and Combustion • Corrosion – Oxidation of a metal by oxygen – Widespread and costly problem • Billions of dollars a year are spent in the United States for steel alone 4 Fe + 3 O 2 + 3 H 2 O 2 Fe 2 O 3 3 H 2 O © 2014 Pearson Education, Inc.

11. 8 Oxygen Is Responsible for Corrosion and Combustion • Other Type of Corrosion – Aluminum oxidizes to Al 2 O 3 and creates a protective coating over the metallic aluminum – Zinc is used in galvanization as a sacrificial metal coating © 2014 Pearson Education, Inc.

11. 8 Oxygen Is Responsible for Corrosion and Combustion • Cathodic Protection – Use of a more easily oxidized metal to protect a metallic structure © 2014 Pearson Education, Inc.

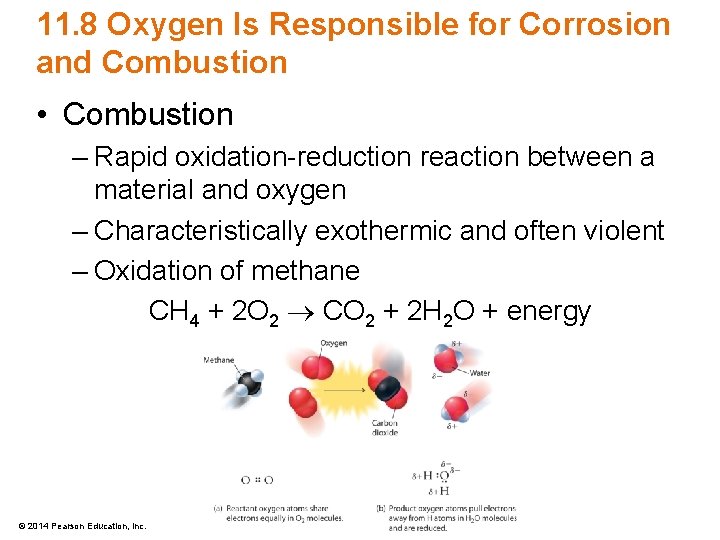
11. 8 Oxygen Is Responsible for Corrosion and Combustion • Combustion – Rapid oxidation-reduction reaction between a material and oxygen – Characteristically exothermic and often violent – Oxidation of methane CH 4 + 2 O 2 CO 2 + 2 H 2 O + energy © 2014 Pearson Education, Inc.