Lecture Power Points Chapter 29 Physics Principles with








- Slides: 49

Lecture Power. Points Chapter 29 Physics: Principles with Applications, 7 th edition Giancoli © 2014 Pearson Education, Inc. This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching their courses and assessing student learning. Dissemination or sale of any part of this work (including on the World Wide Web) will destroy the integrity of the work and is not permitted. The work and materials from it should never be made available to students except by instructors using the accompanying text in their classes. All recipients of this work are expected to abide by these restrictions and to honor the intended pedagogical purposes and the needs of other instructors who rely on these materials.

Chapter 29 Molecules and Solids © 2014 Pearson Education, Inc.

Contents of Chapter 29 • Bonding in Molecules • Potential-Energy Diagrams for Molecules • Weak (van der Waals) Bonds • Molecular Spectra • Bonding in Solids • Free-Electron Theory of Metals; Fermi Energy • Band Theory of Solids © 2014 Pearson Education, Inc.

Contents of Chapter 29 • Semiconductors and Doping • Semiconductor Diodes, LEDs, OLEDs • Transistors: Bipolar and MOSFETs • Integrated Circuits, 22 -nm Technology © 2014 Pearson Education, Inc.

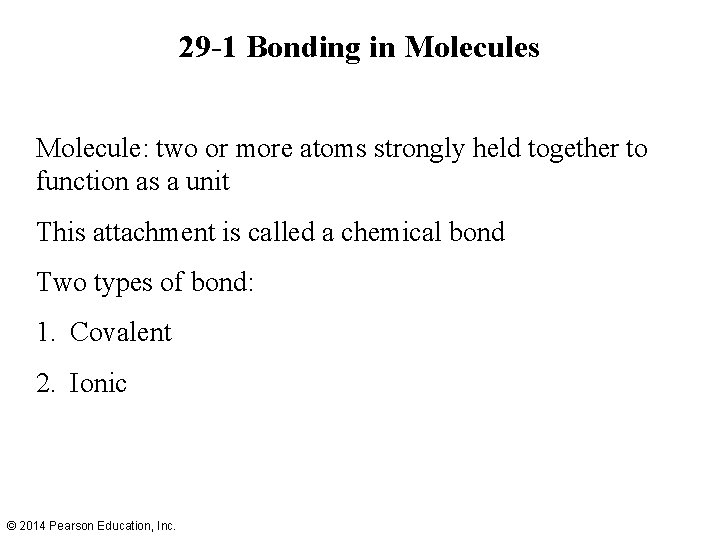
29 -1 Bonding in Molecules Molecule: two or more atoms strongly held together to function as a unit This attachment is called a chemical bond Two types of bond: 1. Covalent 2. Ionic © 2014 Pearson Education, Inc.

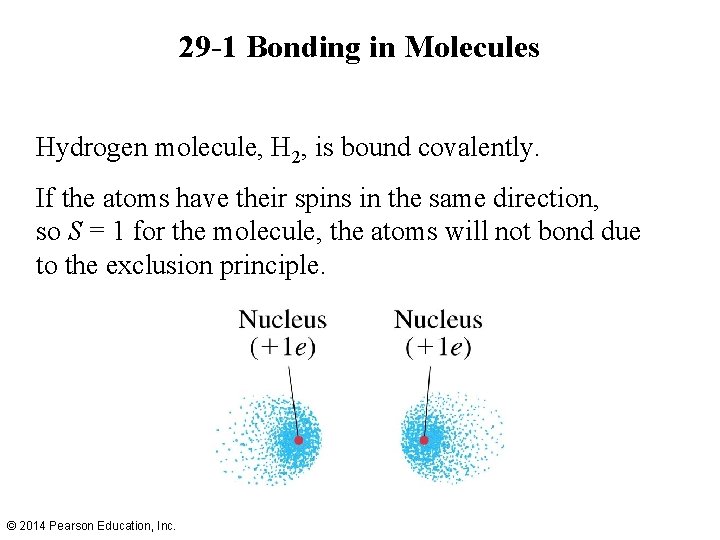
29 -1 Bonding in Molecules Hydrogen molecule, H 2, is bound covalently. If the atoms have their spins in the same direction, so S = 1 for the molecule, the atoms will not bond due to the exclusion principle. © 2014 Pearson Education, Inc.

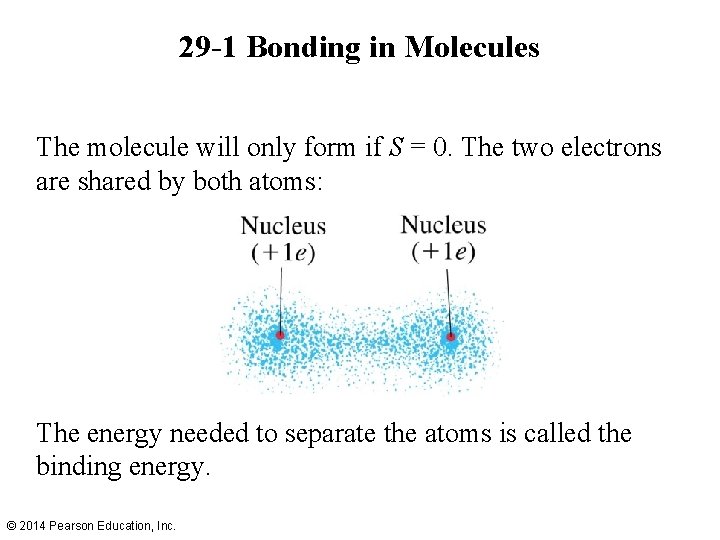
29 -1 Bonding in Molecules The molecule will only form if S = 0. The two electrons are shared by both atoms: The energy needed to separate the atoms is called the binding energy. © 2014 Pearson Education, Inc.

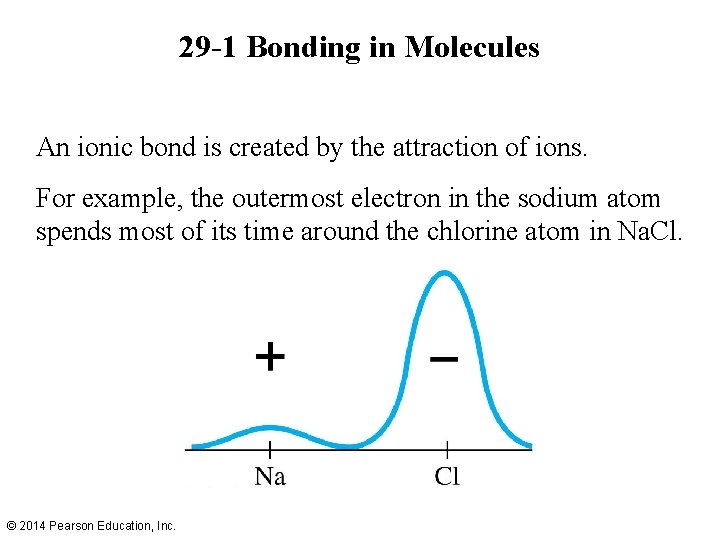
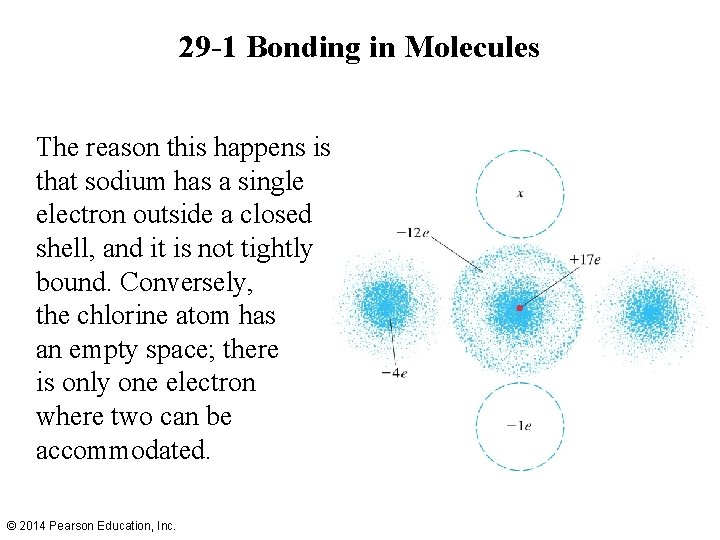
29 -1 Bonding in Molecules An ionic bond is created by the attraction of ions. For example, the outermost electron in the sodium atom spends most of its time around the chlorine atom in Na. Cl. © 2014 Pearson Education, Inc.

29 -1 Bonding in Molecules The reason this happens is that sodium has a single electron outside a closed shell, and it is not tightly bound. Conversely, the chlorine atom has an empty space; there is only one electron where two can be accommodated. © 2014 Pearson Education, Inc.

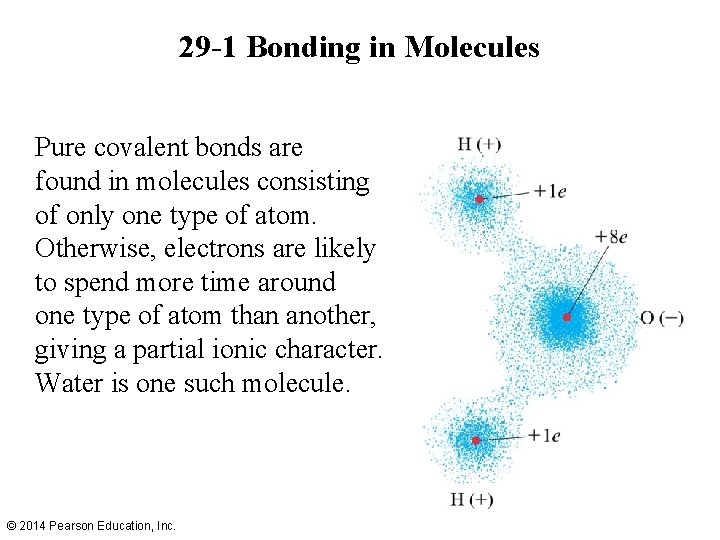
29 -1 Bonding in Molecules Pure covalent bonds are found in molecules consisting of only one type of atom. Otherwise, electrons are likely to spend more time around one type of atom than another, giving a partial ionic character. Water is one such molecule. © 2014 Pearson Education, Inc.

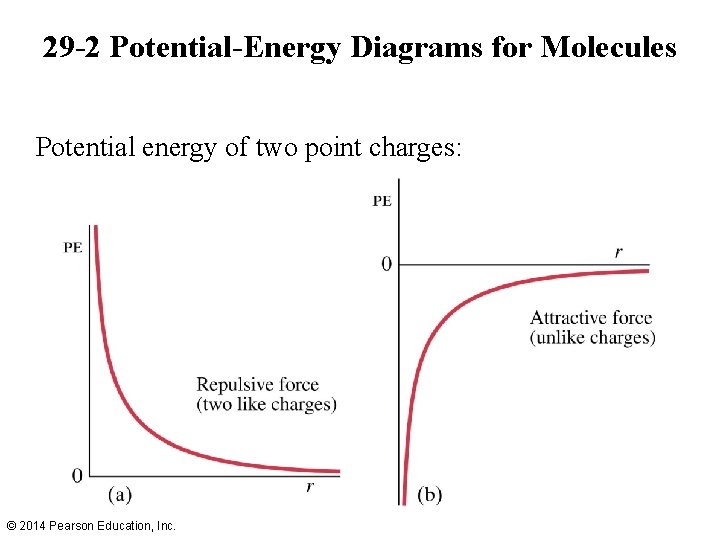
29 -2 Potential-Energy Diagrams for Molecules Potential energy of two point charges: © 2014 Pearson Education, Inc.

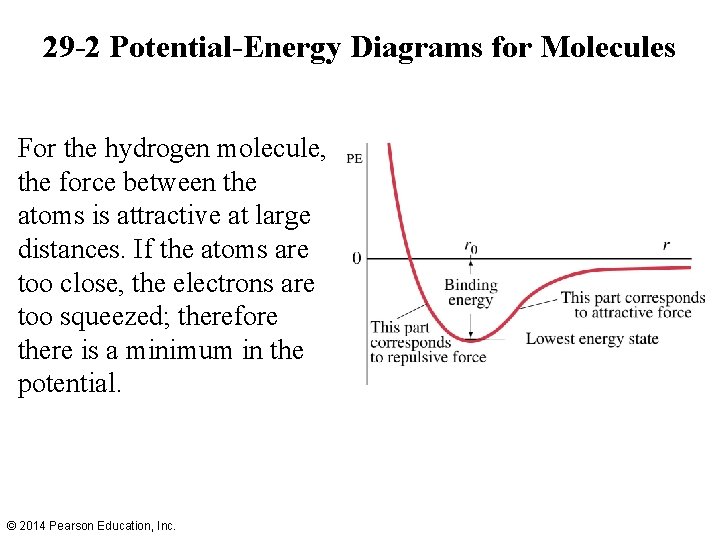
29 -2 Potential-Energy Diagrams for Molecules For the hydrogen molecule, the force between the atoms is attractive at large distances. If the atoms are too close, the electrons are too squeezed; therefore there is a minimum in the potential. © 2014 Pearson Education, Inc.

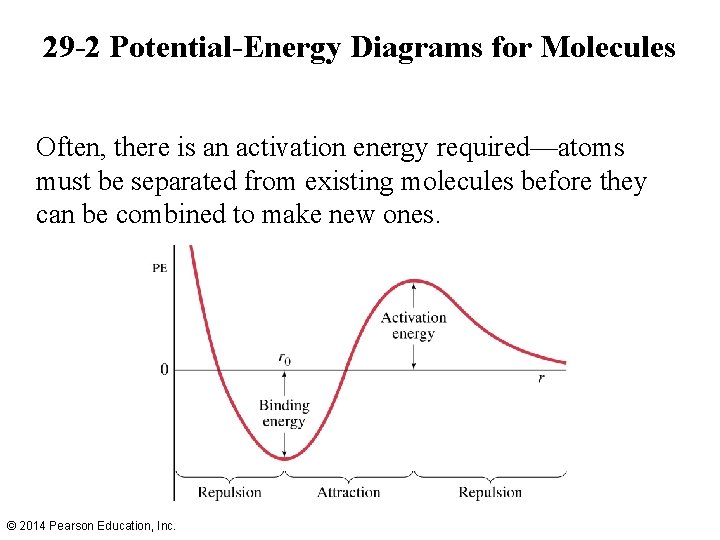
29 -2 Potential-Energy Diagrams for Molecules Often, there is an activation energy required—atoms must be separated from existing molecules before they can be combined to make new ones. © 2014 Pearson Education, Inc.

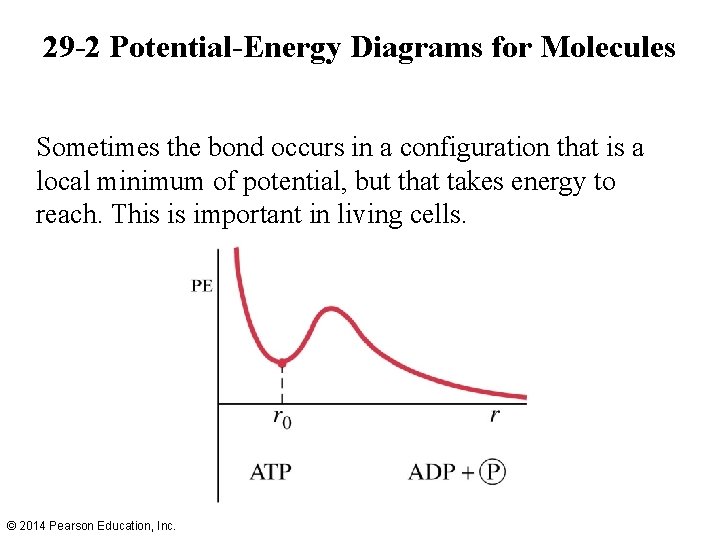
29 -2 Potential-Energy Diagrams for Molecules Sometimes the bond occurs in a configuration that is a local minimum of potential, but that takes energy to reach. This is important in living cells. © 2014 Pearson Education, Inc.

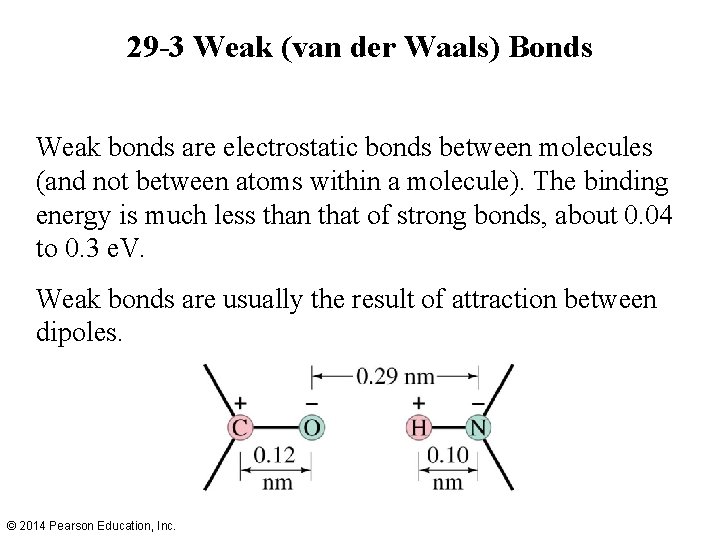
29 -3 Weak (van der Waals) Bonds Weak bonds are electrostatic bonds between molecules (and not between atoms within a molecule). The binding energy is much less than that of strong bonds, about 0. 04 to 0. 3 e. V. Weak bonds are usually the result of attraction between dipoles. © 2014 Pearson Education, Inc.

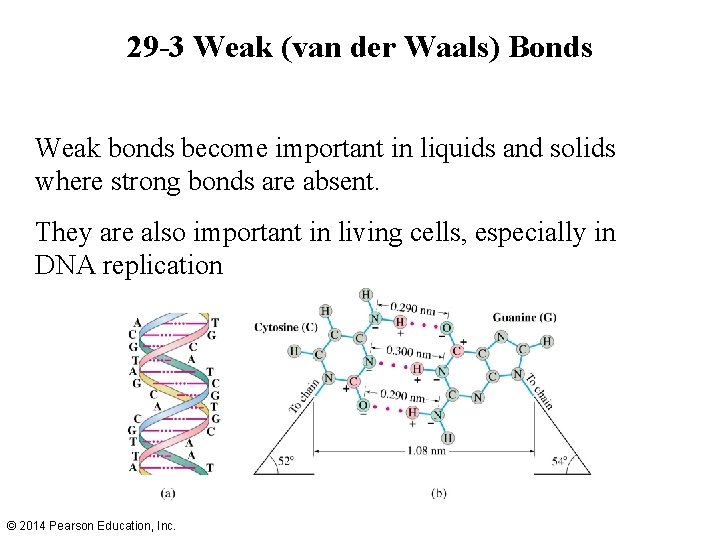
29 -3 Weak (van der Waals) Bonds Weak bonds become important in liquids and solids where strong bonds are absent. They are also important in living cells, especially in DNA replication © 2014 Pearson Education, Inc.

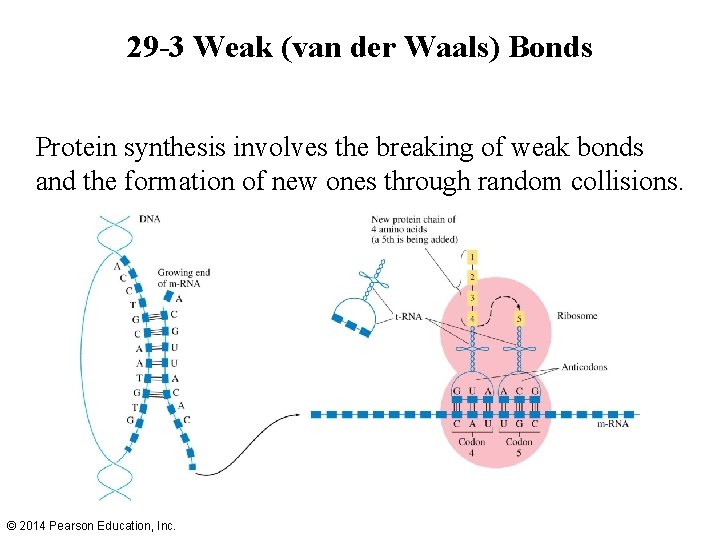
29 -3 Weak (van der Waals) Bonds Protein synthesis involves the breaking of weak bonds and the formation of new ones through random collisions. © 2014 Pearson Education, Inc.

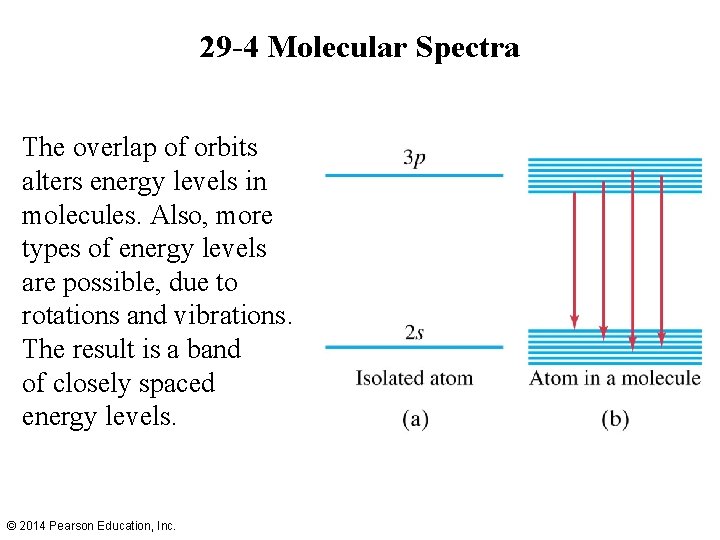
29 -4 Molecular Spectra The overlap of orbits alters energy levels in molecules. Also, more types of energy levels are possible, due to rotations and vibrations. The result is a band of closely spaced energy levels. © 2014 Pearson Education, Inc.

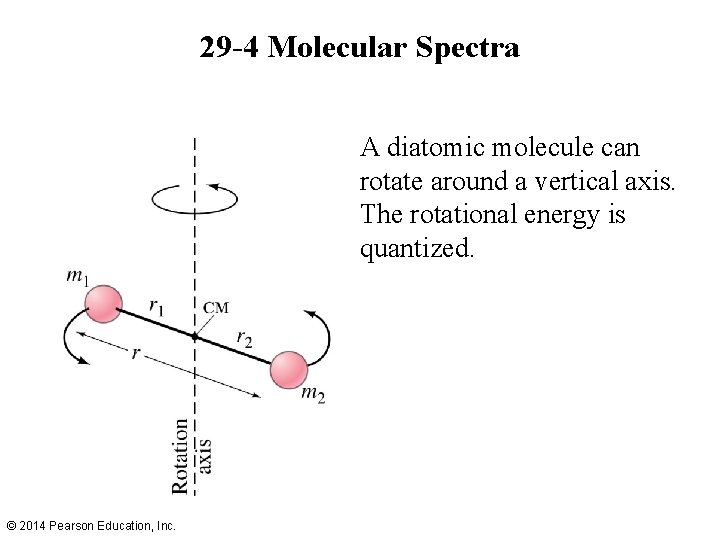
29 -4 Molecular Spectra A diatomic molecule can rotate around a vertical axis. The rotational energy is quantized. © 2014 Pearson Education, Inc.

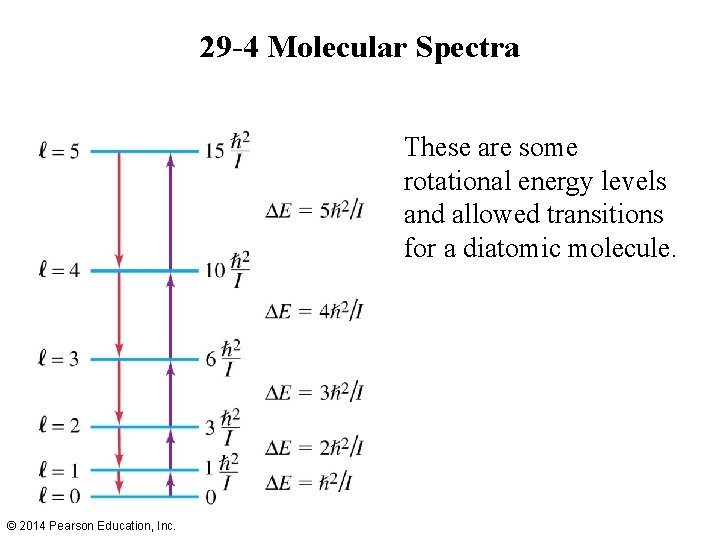
29 -4 Molecular Spectra These are some rotational energy levels and allowed transitions for a diatomic molecule. © 2014 Pearson Education, Inc.

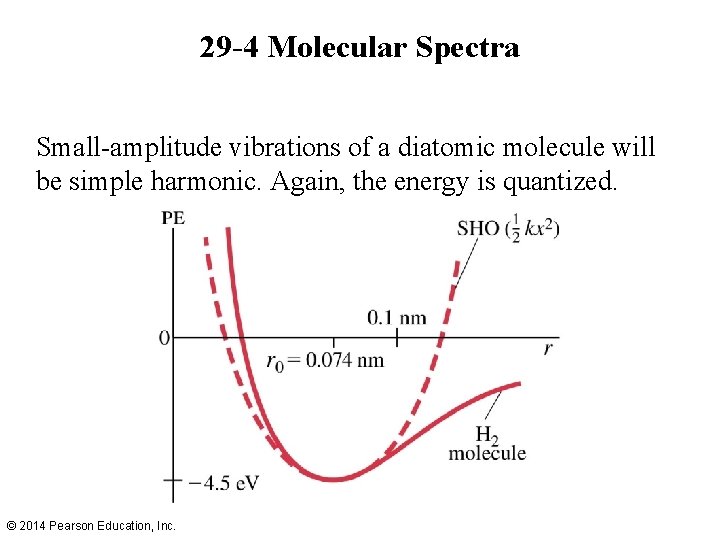
29 -4 Molecular Spectra Small-amplitude vibrations of a diatomic molecule will be simple harmonic. Again, the energy is quantized. © 2014 Pearson Education, Inc.

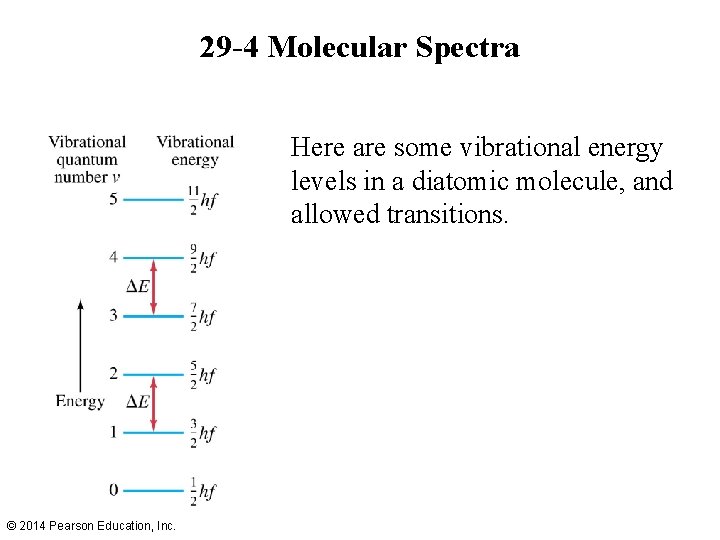
29 -4 Molecular Spectra Here are some vibrational energy levels in a diatomic molecule, and allowed transitions. © 2014 Pearson Education, Inc.

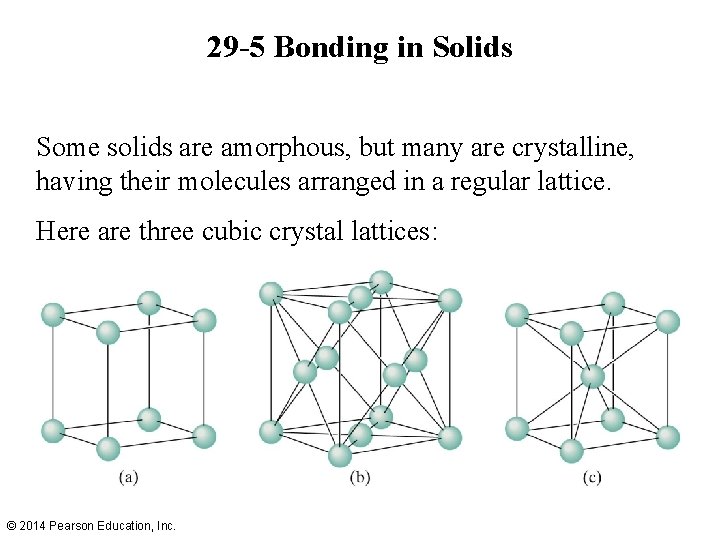
29 -5 Bonding in Solids Some solids are amorphous, but many are crystalline, having their molecules arranged in a regular lattice. Here are three cubic crystal lattices: © 2014 Pearson Education, Inc.

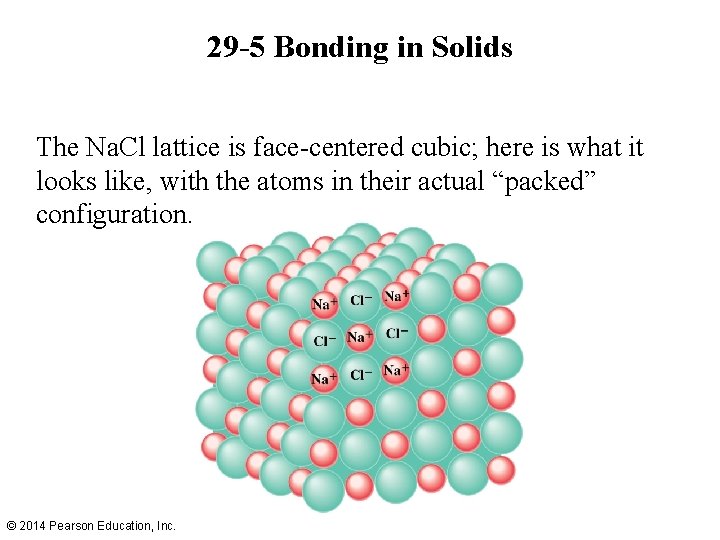
29 -5 Bonding in Solids The Na. Cl lattice is face-centered cubic; here is what it looks like, with the atoms in their actual “packed” configuration. © 2014 Pearson Education, Inc.

29 -5 Bonding in Solids Metallic bonds, where electrons are shared by all atoms in the metal, are neither ionic or covalent. The binding energy of metallic bonds is slightly weaker than that of ionic or covalent bonds—about 1 to 3 e. V—but they are still strong bonds. © 2014 Pearson Education, Inc.

29 -6 Free-Electron Theory of Metals; Fermi Energy The free-electron theory treats the electrons in a metal as an ideal gas, with the extra constraint of the exclusion principle—no two electrons can be in the same quantum state. © 2014 Pearson Education, Inc.

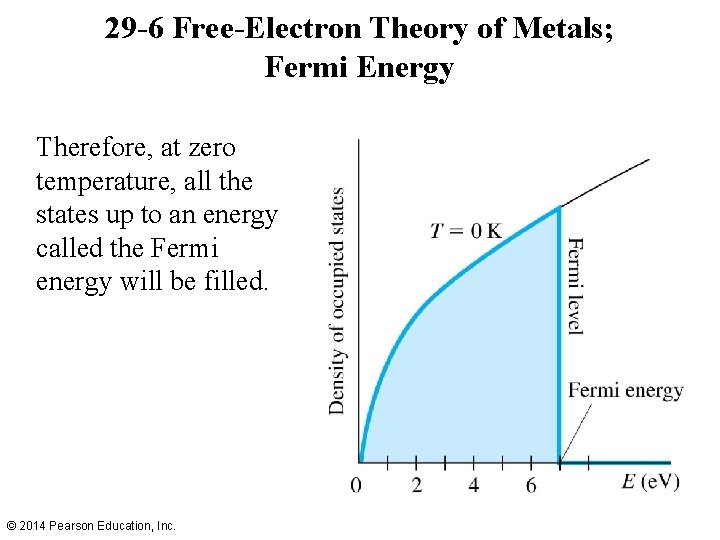
29 -6 Free-Electron Theory of Metals; Fermi Energy Therefore, at zero temperature, all the states up to an energy called the Fermi energy will be filled. © 2014 Pearson Education, Inc.

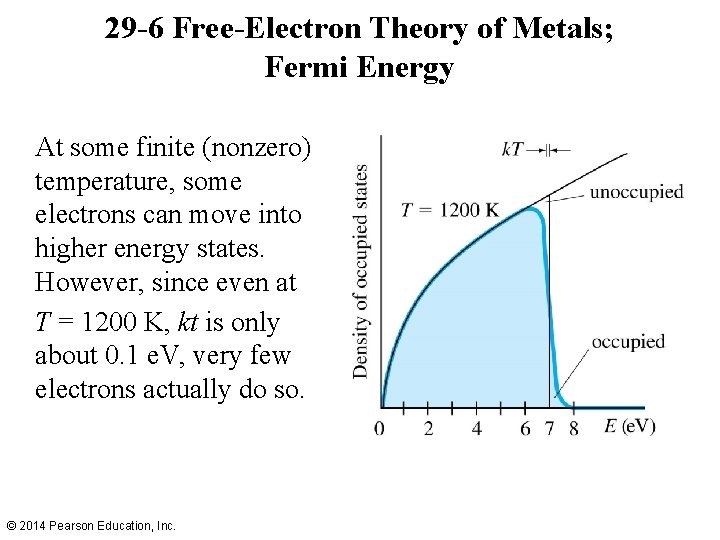
29 -6 Free-Electron Theory of Metals; Fermi Energy At some finite (nonzero) temperature, some electrons can move into higher energy states. However, since even at T = 1200 K, kt is only about 0. 1 e. V, very few electrons actually do so. © 2014 Pearson Education, Inc.

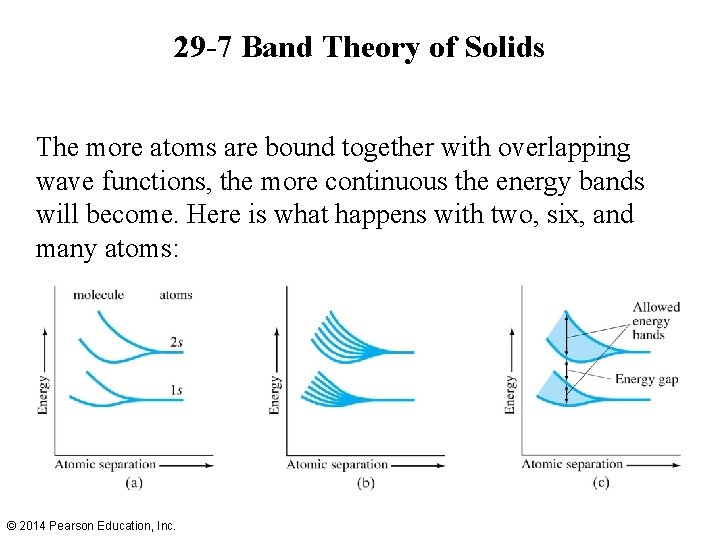
29 -7 Band Theory of Solids The more atoms are bound together with overlapping wave functions, the more continuous the energy bands will become. Here is what happens with two, six, and many atoms: © 2014 Pearson Education, Inc.

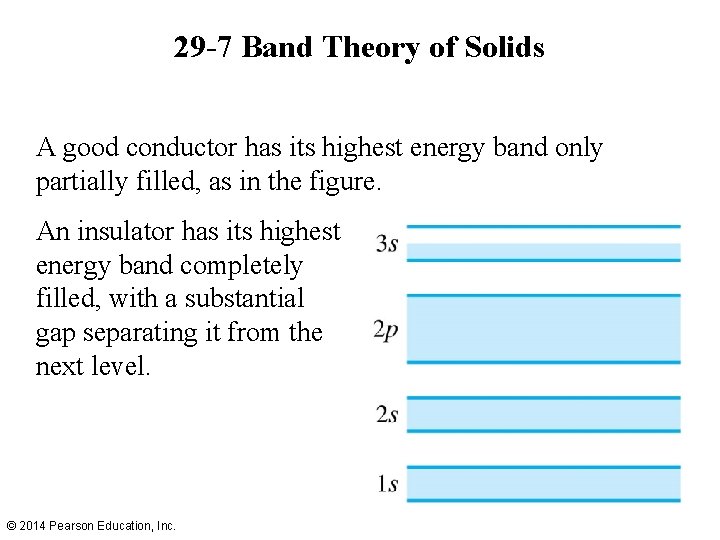
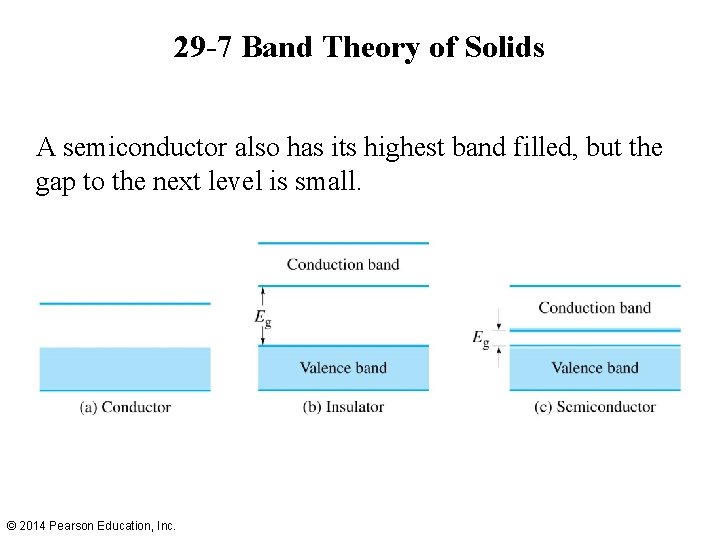
29 -7 Band Theory of Solids A good conductor has its highest energy band only partially filled, as in the figure. An insulator has its highest energy band completely filled, with a substantial gap separating it from the next level. © 2014 Pearson Education, Inc.

29 -7 Band Theory of Solids A semiconductor also has its highest band filled, but the gap to the next level is small. © 2014 Pearson Education, Inc.

29 -8 Semiconductors and Doping The most common semiconductors in use are silicon and germanium. A tiny amount of impurity gives the semiconductor useful properties—this is called doping. The doped semiconductor becomes slightly conducting; the conductivity can be controlled with great precision. © 2014 Pearson Education, Inc.

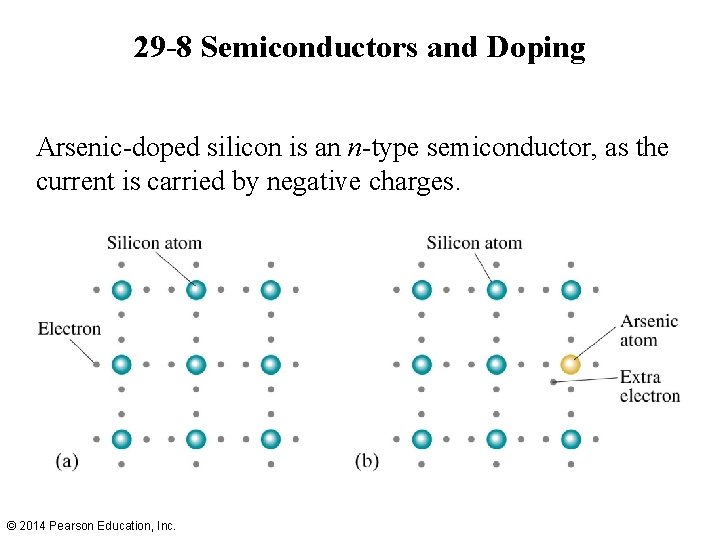
29 -8 Semiconductors and Doping Arsenic-doped silicon is an n-type semiconductor, as the current is carried by negative charges. © 2014 Pearson Education, Inc.

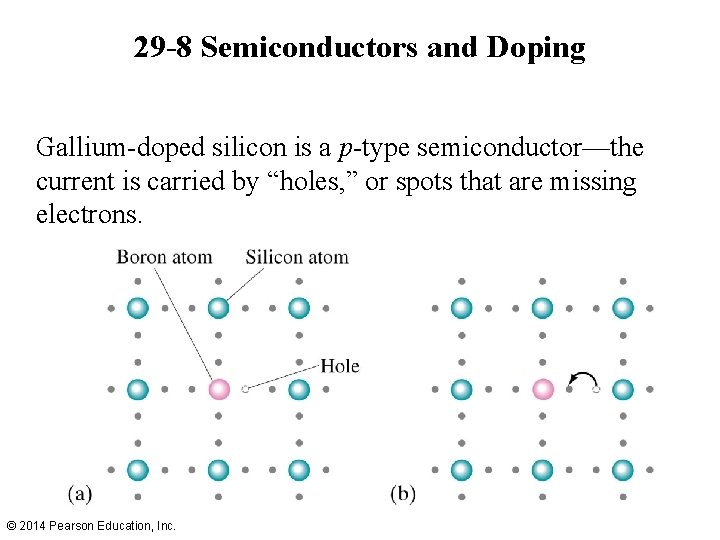
29 -8 Semiconductors and Doping Gallium-doped silicon is a p-type semiconductor—the current is carried by “holes, ” or spots that are missing electrons. © 2014 Pearson Education, Inc.

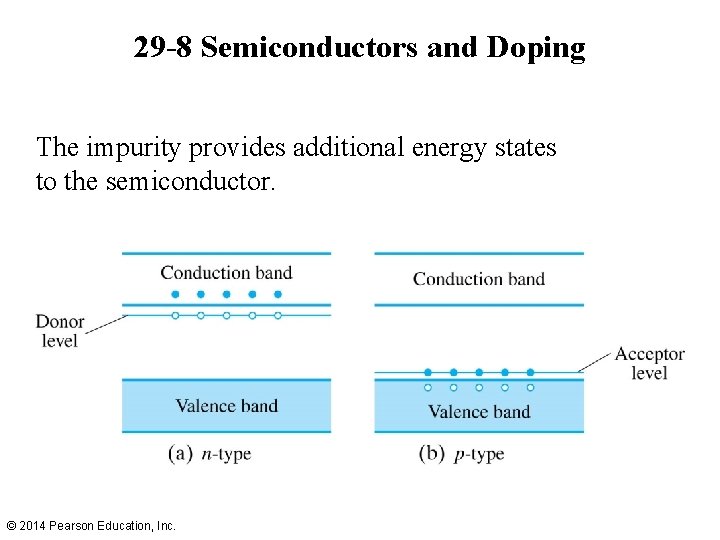
29 -8 Semiconductors and Doping The impurity provides additional energy states to the semiconductor. © 2014 Pearson Education, Inc.

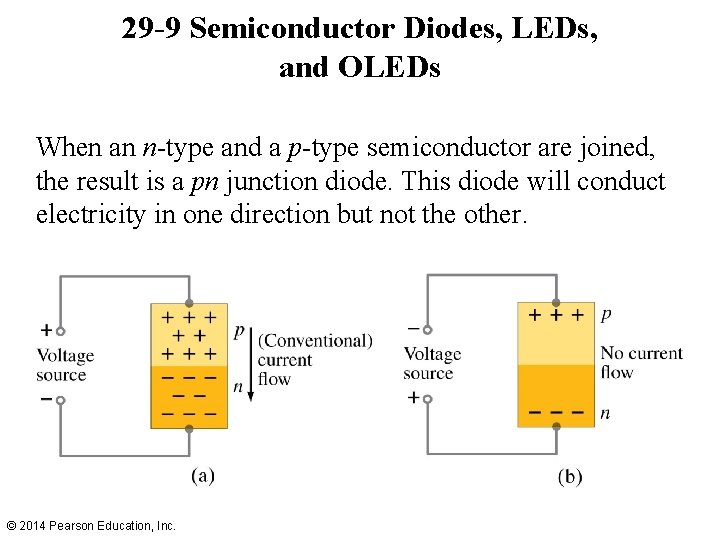
29 -9 Semiconductor Diodes, LEDs, and OLEDs When an n-type and a p-type semiconductor are joined, the result is a pn junction diode. This diode will conduct electricity in one direction but not the other. © 2014 Pearson Education, Inc.

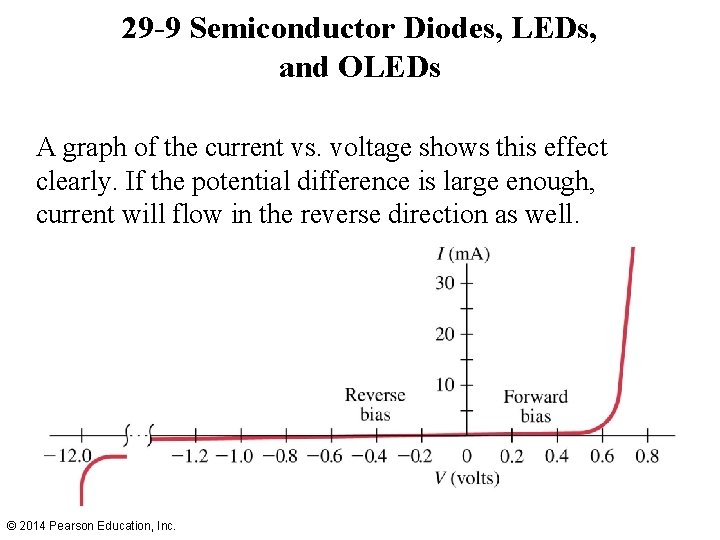
29 -9 Semiconductor Diodes, LEDs, and OLEDs A graph of the current vs. voltage shows this effect clearly. If the potential difference is large enough, current will flow in the reverse direction as well. © 2014 Pearson Education, Inc.

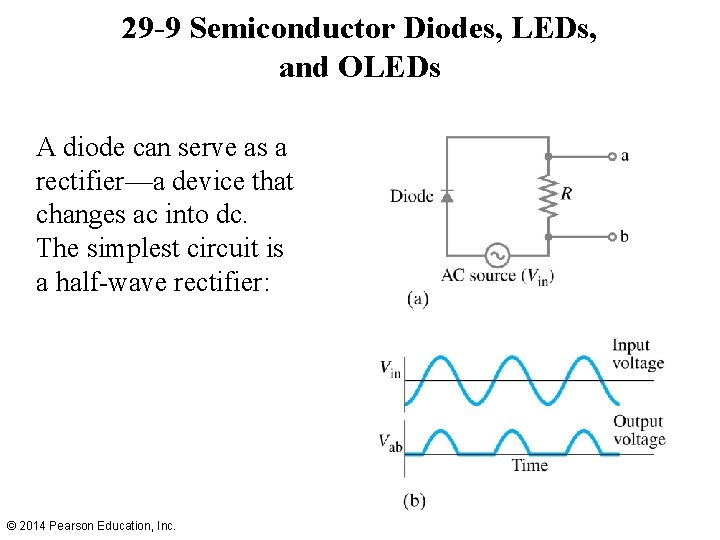
29 -9 Semiconductor Diodes, LEDs, and OLEDs A diode can serve as a rectifier—a device that changes ac into dc. The simplest circuit is a half-wave rectifier: © 2014 Pearson Education, Inc.

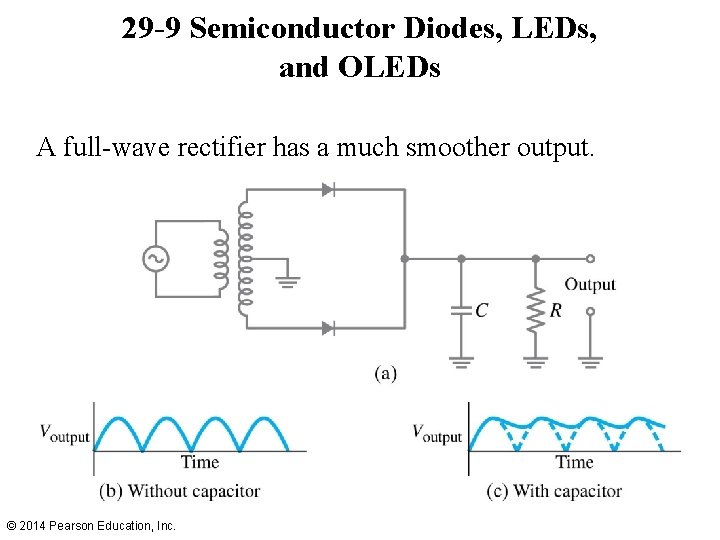
29 -9 Semiconductor Diodes, LEDs, and OLEDs A full-wave rectifier has a much smoother output. © 2014 Pearson Education, Inc.

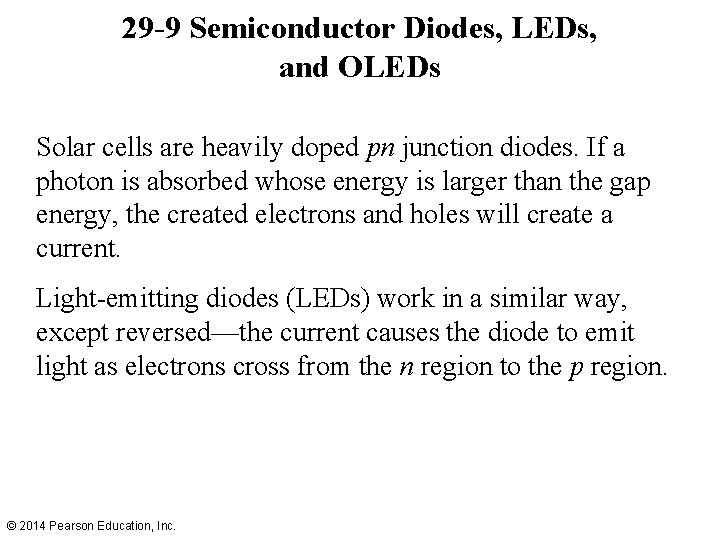
29 -9 Semiconductor Diodes, LEDs, and OLEDs Solar cells are heavily doped pn junction diodes. If a photon is absorbed whose energy is larger than the gap energy, the created electrons and holes will create a current. Light-emitting diodes (LEDs) work in a similar way, except reversed—the current causes the diode to emit light as electrons cross from the n region to the p region. © 2014 Pearson Education, Inc.

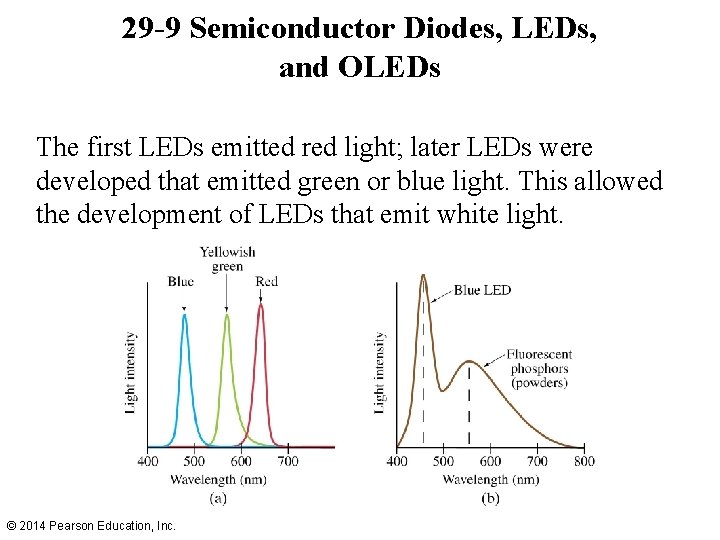
29 -9 Semiconductor Diodes, LEDs, and OLEDs The first LEDs emitted red light; later LEDs were developed that emitted green or blue light. This allowed the development of LEDs that emit white light. © 2014 Pearson Education, Inc.

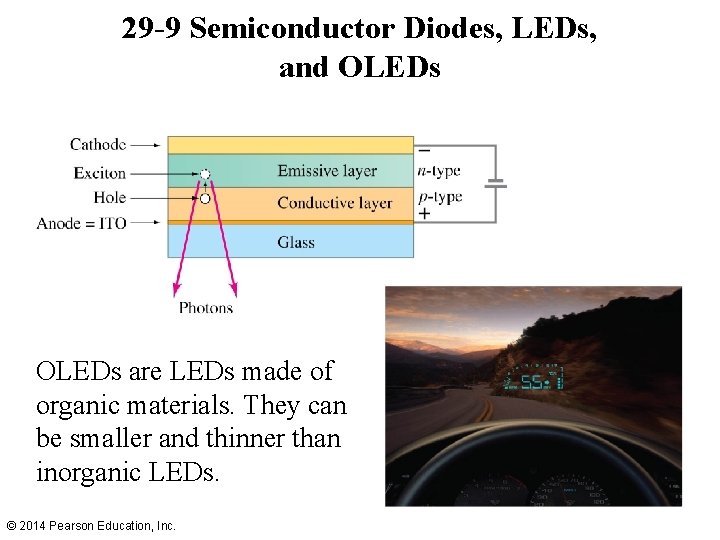
29 -9 Semiconductor Diodes, LEDs, and OLEDs are LEDs made of organic materials. They can be smaller and thinner than inorganic LEDs. © 2014 Pearson Education, Inc.

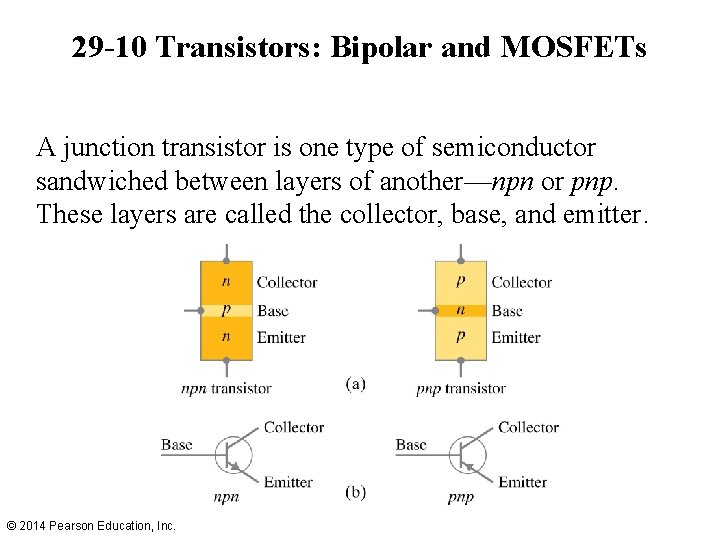
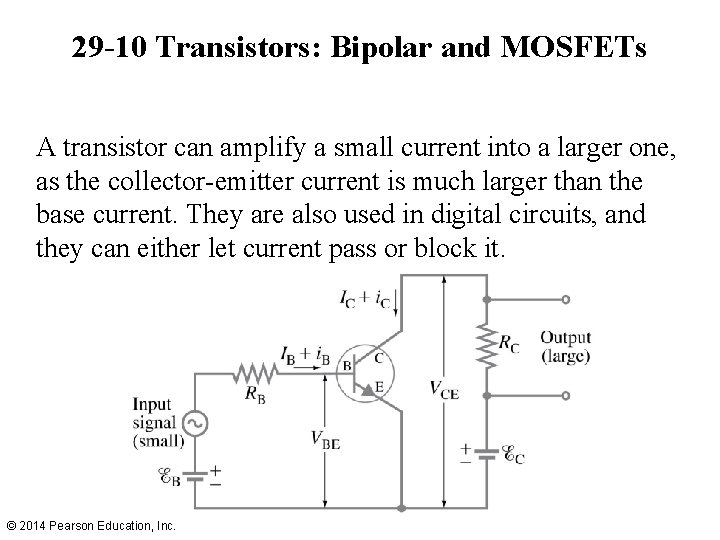
29 -10 Transistors: Bipolar and MOSFETs A junction transistor is one type of semiconductor sandwiched between layers of another—npn or pnp. These layers are called the collector, base, and emitter. © 2014 Pearson Education, Inc.

29 -10 Transistors: Bipolar and MOSFETs A transistor can amplify a small current into a larger one, as the collector-emitter current is much larger than the base current. They are also used in digital circuits, and they can either let current pass or block it. © 2014 Pearson Education, Inc.

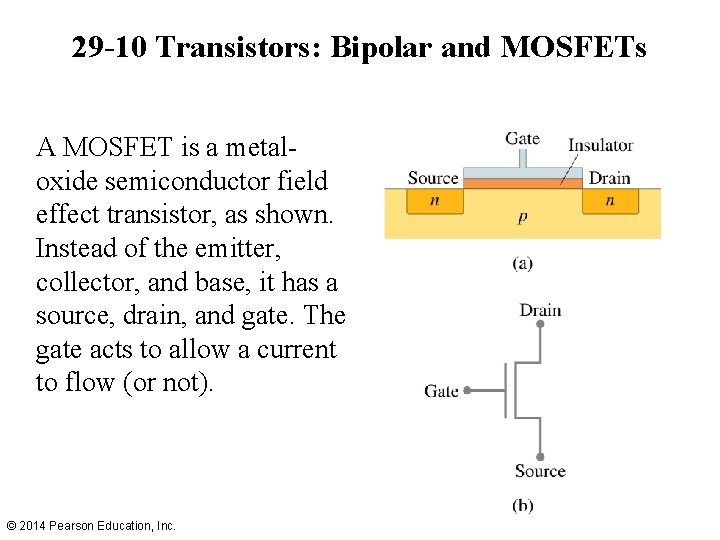
29 -10 Transistors: Bipolar and MOSFETs A MOSFET is a metaloxide semiconductor field effect transistor, as shown. Instead of the emitter, collector, and base, it has a source, drain, and gate. The gate acts to allow a current to flow (or not). © 2014 Pearson Education, Inc.

29 -11 Integrated Circuits, 22 -nm Technology Integrated circuits consist of large wafers of silicon with tiny impurities injected in a pattern. These impurities create diodes, transistors, resistors, and wires. A single integrated circuit chip can contain billions of transistors and other circuit elements, at a scale (as of publication) of 22 nm. © 2014 Pearson Education, Inc.

Summary of Chapter 29 • Molecules form either covalent or ionic bonds • Electron wave functions overlap • Weak (van der Waals) bonds are dipole attractions between molecules • Energy levels in molecules are altered • Additional energy levels are possible, corresponding to rotational and vibrational states • Energy levels become closely-spaced bands © 2014 Pearson Education, Inc.

Summary of Chapter 29 • Rotational energy levels are quantized • Vibrational energy levels are quantized too • Solids can be bound by ionic, covalent, or metallic bonds • Electron energy levels in crystals are bands, with gaps in between • In conductors, the highest band is partially full • In insulators, the highest band is completely full, and there is a large gap to the next band © 2014 Pearson Education, Inc.

Summary of Chapter 29 • In semiconductors, the highest band is completely full but the energy gap is much smaller • In doped semiconductors, small amounts of impurities allow current to be very precisely controlled • Doped semiconductors can be either p-type or n-type • A diode is a pn junction • A transistor is a pnp or npn junction © 2014 Pearson Education, Inc.