Lecture Outlines Chapter 17 Environment The Science behind

Lecture Outlines Chapter 17 Environment: The Science behind the Stories 4 th Edition Withgott/Brennan © 2011 Pearson Education, Inc.

This lecture will help you understand: • The Earth’s atmosphere • Weather, climate, and atmospheric conditions • Outdoor pollution and solutions • Stratospheric ozone depletion • Acidic deposition and consequences • Indoor air pollution and solutions © 2011 Pearson Education, Inc.

Notes HW • Write each slide title on the left side of the paper • Write provided information on the right side of the paper • If there are slides with Objectives or “this lecture will help you understand” you do NOT need to write these. • Define any words or answer any questions or fill in the blanks when something appears in red. - Sometimes it is a question linked to a website you should view • Sometime there are comments written in purple. You do not need to write these. They are just my personal commentary • Be prepared to answer questions at the end. © 2011 Pearson Education, Inc.

Central Case: L. A. and its sister cities struggle for a breath of clean air • Vehicles caused smog in Los Angeles from 1970 s to 1990 s • Policies and technologies improved its air qualities - But its “sister cities” are not as clean • 3, 600/month die in Tehran from air pollution - Old cars use cheap gas - Topography, immigration, etc. © 2011 Pearson Education, Inc.
The atmosphere http: //www. youtube. com/watch? v=Uc. Wpk. WBX 04 E • What image was most powerful for you from the 2 minute video? • Atmosphere ? - Absorbs radiation and moderates climate - 78% N 2, 21% O 2 • Human activity is changing the amount of some gases - CO 2, methane (CH 4), ozone (O 3) © 2011 Pearson Education, Inc.

The first two layers of the atmosphere • Troposphere ? - Air for breathing, weather - The air gets colder with altitude • Stratosphere ? - Becomes warmer with altitude - Contains UV radiation-blocking ozone • Mesosphere ? - Extremely low air pressure - Temperatures decrease with altitude • Thermosphere ? © 2011 Pearson Education, Inc.
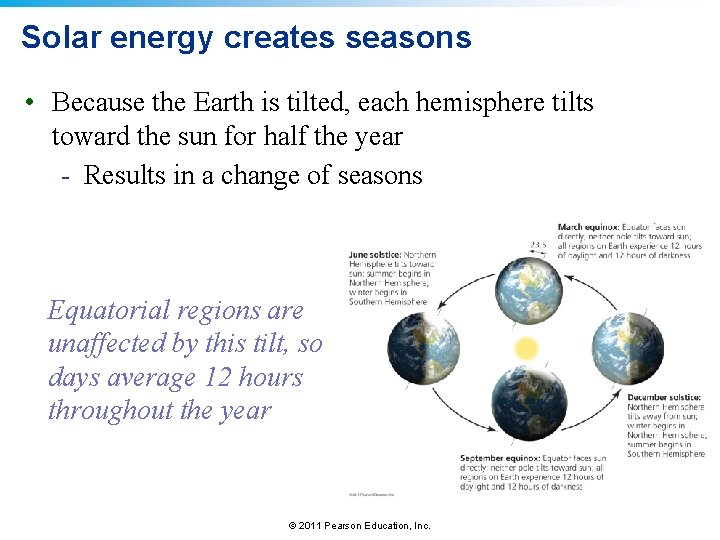
Solar energy creates seasons • Because the Earth is tilted, each hemisphere tilts toward the sun for half the year - Results in a change of seasons Equatorial regions are unaffected by this tilt, so days average 12 hours throughout the year © 2011 Pearson Education, Inc.
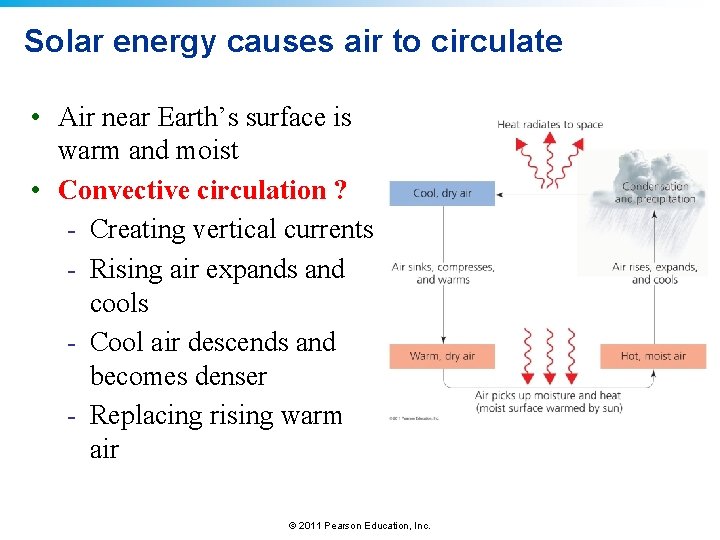
Solar energy causes air to circulate • Air near Earth’s surface is warm and moist • Convective circulation ? - Creating vertical currents - Rising air expands and cools - Cool air descends and becomes denser - Replacing rising warm air Convection influences weather and climate © 2011 Pearson Education, Inc.

The atmosphere drives weather and climate • Weather ? • Climate ? • Mark Twain said, “Climate is what we expect; weather is what we get” Really? ? ? © 2011 Pearson Education, Inc.
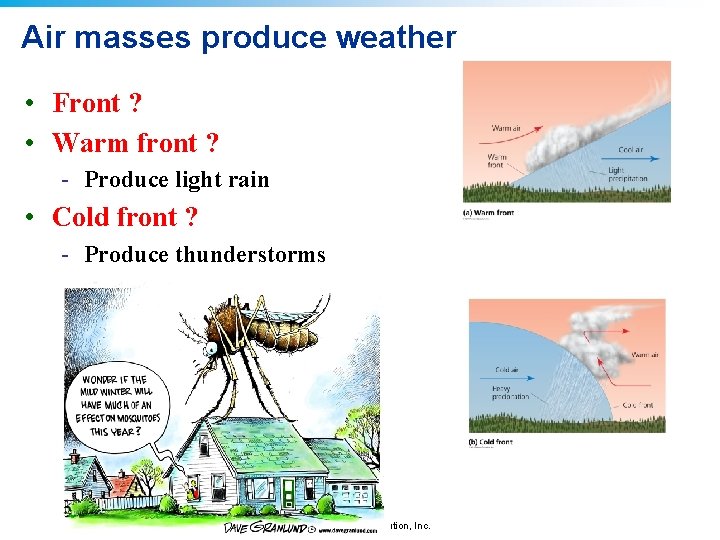
Air masses produce weather • Front ? • Warm front ? - Produce light rain • Cold front ? - Produce thunderstorms © 2011 Pearson Education, Inc.
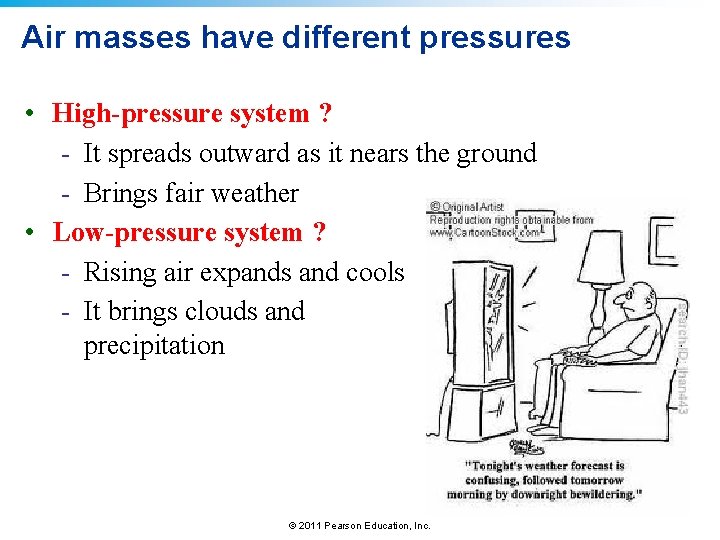
Air masses have different pressures • High-pressure system ? - It spreads outward as it nears the ground - Brings fair weather • Low-pressure system ? - Rising air expands and cools - It brings clouds and precipitation © 2011 Pearson Education, Inc.
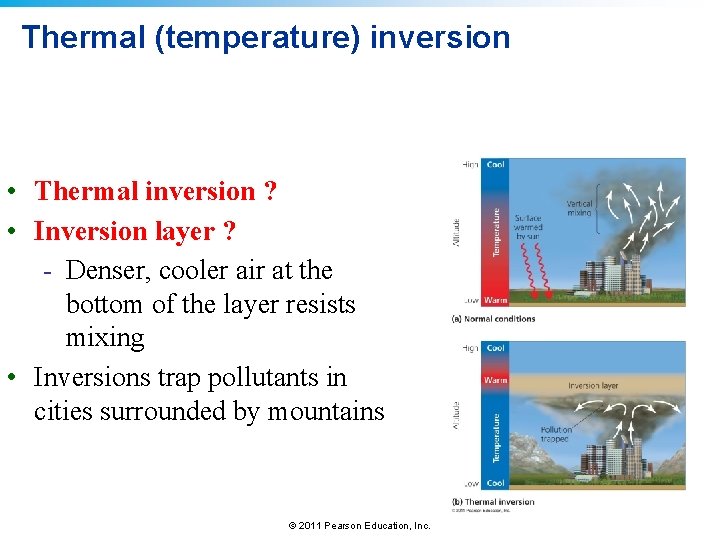
Thermal (temperature) inversion • Thermal inversion ? • Inversion layer ? - Denser, cooler air at the bottom of the layer resists mixing • Inversions trap pollutants in cities surrounded by mountains © 2011 Pearson Education, Inc.
Global wind patterns • Coriolis effect ? - Results in curving global wind patterns called the doldrums, trade winds, and westerlies • Doldrums = a region near the equator with few winds • Trade winds = between the equator and _? __ degrees - Blow from east to west - Weaken periodically, leading to El Niño conditions • Westerlies = from _? __ to _? __ degrees latitude - Blow from west to east © 2011 Pearson Education, Inc.

Storms pose hazards • Atmospheric conditions can produce dangerous storms • Hurricanes ? - Warm, moist air over the topical oceans rises • Typhoons (cyclones) ? - Drawing up huge amounts of water vapor lading to heavy rains • Tornadoes ? - form when warm air meets cold air - Quickly rising warm air forms a powerful convective current (spinning funnel) © 2011 Pearson Education, Inc.

Natural sources pollute too! • Volcanoes • Fires • Dust storms © 2011 Pearson Education, Inc.

We create outdoor air pollution • Point sources = specific spots where large quantities of pollutants are discharged (power plants and factories) • Non-point sources = more diffuse, consisting of many small sources (automobiles) • Primary pollutants ? - 2 examples? • Secondary pollutants ? - 2 examples? • Residence time ? Tiananmen Square, China displays a screen with a clear sky on a typically smoggy day © 2011 Pearson Education, Inc.

Legislation addresses pollution • The Clean Air Act of 1970 & 1990 - Set standards for air quality, limits on emissions - Provided funds for pollution-control research - Allowed citizens to sue parties violating the standards - Introduced emissions trading for sulfur dioxide - The EPA sets nationwide standards for emissions and concentrations of toxic pollutants - Total emissions of the six monitored pollutants have declined 60% since the Clean Air Act of 1970 © 2011 Pearson Education, Inc.
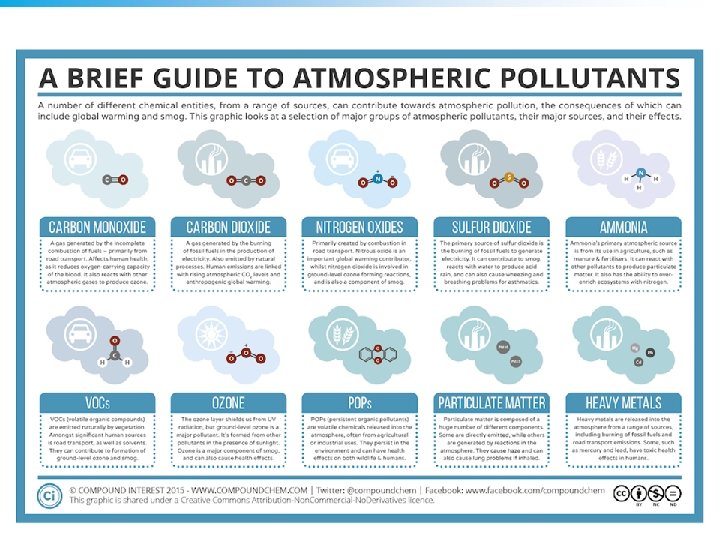
Major Air Pollutants- SPLONC • Sulfur dioxide (SO 2) and sulfuric acid (SOx): - About one-third of SO 2 in the troposphere occurs naturally through the sulfur cycle. - Two-thirds come from human sources, mostly combustion (S+ O 2 SO 2) of sulfur -containing coal and from oil refining and smelting of sulfide ores. - SO 2 in the atmosphere can be converted to sulfuric acid (H 2 SO 4) and sulfate salts (SO 42 -) that return to earth as a component of acid deposition. © 2011 Pearson Education, Inc.
Major Air Pollutants- SPLONC • suspended Particulate matter (SPM): - Consists of a variety of solid particles and liquid droplets small and light enough to remain suspended in the air. - The most harmful forms of SPM are fine particles (PM-10, with an average diameter < 10 micrometers) and ultrafine particles (PM-2. 5). © 2011 Pearson Education, Inc.

Major Air Pollutants- SPLONC • Lead - Solid toxic medal and its compounds, emitted as a particulate - Found in paint, pipes, batteries, leaded gasoline -(used to boost octane, US ban began in 1973 -1996) - Accumulates in the brain and can cause cognitive development problems, digestive disorders, and has been shown to cause cancer in test animals © 2011 Pearson Education, Inc.

Major Air Pollutants- SPLONC • Ozone (O 3): - Is a highly reactive gas that is a major component of photochemical smog. - Results from interaction of sunlight, heat, Nox, Vocs - It can - Cause and aggravate respiratory illness. - Can aggravate heart disease. - Damage plants, rubber in tires, fabrics, and paints. © 2011 Pearson Education, Inc.

Major Air Pollutants- SPLONC • Nitrogen oxides and nitric acid: - Nitrogen oxide (NO) forms when nitrogen and oxygen gas in air react at the high-combustion temperatures in automobile engines and coal-burning plants. NO can also form from lightening and certain soil bacteria. - NO reacts with air to form NO 2. - NO 2 reacts with water vapor in the air to form nitric acid (HNO 3) and nitrate salts (NO 3 -) which are components of acid deposition. © 2011 Pearson Education, Inc.
Major Air Pollutants- SPLONC • Carbon oxides: - Carbon monoxide (CO) is a highly toxic gas that forms during the incomplete combustion of carboncontaining materials. - 93% of carbon dioxide (CO 2) in the troposphere occurs as a result of the carbon cycle. - 7% of CO 2 in the troposphere occurs as a result of human activities (mostly burning fossil fuels). - It is not regulated as a pollutant under the U. S. Clean Air Act. © 2011 Pearson Education, Inc.
© 2011 Pearson Education, Inc.
Agencies monitor emissions • State and local agencies monitor, calculate, and report to the EPA the emissions of these pollutants: - Carbon monoxide, sulfur dioxide, particulate matter, lead, and all nitrogen oxides • Tropospheric ozone has no emissions to monitor - It is a secondary pollutant • Agencies monitor - volatile organic compounds (VOCs) ? - PANs ? © 2011 Pearson Education, Inc.
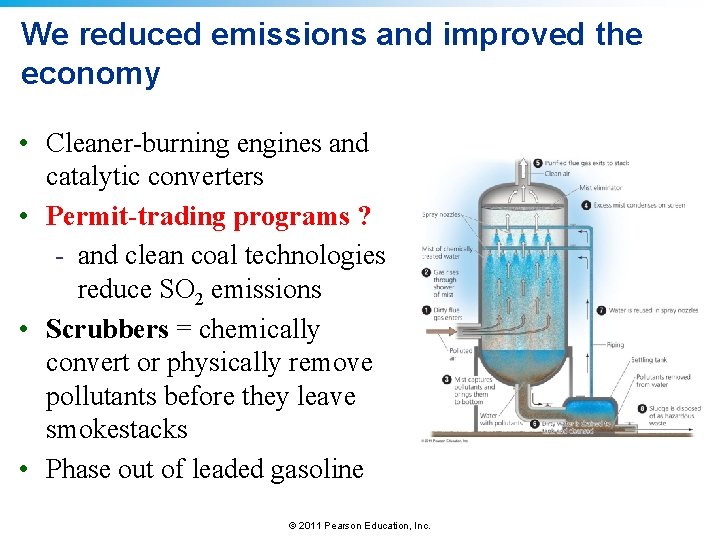
We reduced emissions and improved the economy • Cleaner-burning engines and catalytic converters • Permit-trading programs ? - and clean coal technologies reduce SO 2 emissions • Scrubbers = chemically convert or physically remove pollutants before they leave smokestacks • Phase out of leaded gasoline © 2011 Pearson Education, Inc.

Smog: our most common air quality problem • Photochemical smog ? • Sulfur in burned coal combines with oxygen to form sulfuric acid • Industrial (gray air) smog ? Produced by a series of reactions Formed in hot, sunny cities surrounded by mountains Light-driven reactions of primary pollutants and atmospheric compounds Morning traffic releases NO and What is emissions trading? © 2011 Pearson Education, Inc.

Synthetic chemicals deplete stratospheric ozone • Ozone-depleting substances = human-made chemicals that destroy ozone by splitting its molecules apart • Halocarbons = human-made compounds made from hydrocarbons with added chlorine, bromine, or fluorine • Chlorofluorocarbons (CFCs) = a halocarbon used as refrigerants, in fire extinguishers, in aerosol cans, etc. - Releases chlorine atoms that split ozone • Montreal Protocol = 196 nations agreed to cut CFC production in half by 1998 © 2011 Pearson Education, Inc.

Disney Reality © 2011 Pearson Education, Inc.

Indoor air pollution • Indoor air pollution = in workplaces, schools, and homes - Health effects are greater than from outdoor pollution • Fireplaces • Radon • Tobacco smoke: individual & second-hand • Mold • VOCs- volatile organic compounds - Formaldehyde leaking from pressed wood and insulation irritates mucous membranes and induces skin allergies - Pesticides seep through floors and walls - Are brought in on shoe soles © 2011 Pearson Education, Inc.

Sick building syndrome - a sickness produced by indoor pollution with general and nonspecific symptoms - used to describe a building in which occupants suffer persistent symptoms that disappear when they go outside - Describe this video? http: //www. youtube. com/watch? v=gci. Sp 5 X 40 t 0&feature=results_main&playnext=1&list=PL 2494 D 51 D 8 EE 7536 A © 2011 Pearson Education, Inc.

CH 17 -A 1) About how thick is the Earth’s atmosphere? Name one characteristic of each of the four atmospheric layers. 2) Where is the ozone layer located? How and why is stratospheric ozone beneficial where as tropospheric ozone is harmful? 3) What is the difference between weather and climate? How does solar energy influence weather and climate? 4) Describe a thermal inversion. How do inversions contribute to sever smog episodes like those in London and Los Angeles? 5) How does a primary pollutant differ from a secondary pollutant? Give an example of each. © 2011 Pearson Education, Inc.

CH 17 -B 6) What has happened with concentrations of criteria pollutants in U. S. ambient air in recent decades? What has happened with our emissions of major pollutants? Name two health risks from toxic air pollutants. 7) How does photochemical smog differ from industrial smog? How do the weather and topography influence smog formation? 8) How do chlorofluorocarbons (CFCs) deplete stratospheric ozone? Why is the depletion considered a long-term international problem? What has been done to address this problem? 9) Why are the effects of acid deposition often felt in areas far from where the primary pollutants are produced? List three impacts of acid deposition. 10) Name five common sources of indoor air pollution. For each, describe one way to reduce exposure to the pollutant. What is sick building syndrome? © 2011 Pearson Education, Inc.

Global climate change occurs because increasing concentrations of greenhouse gases in a. b. c. d. e. The troposphere absorb outgoing IR radiation The stratosphere absorb outgoing IR radiation The troposphere absorb incoming UV radiation The stratosphere absorb incoming UV radiation Neither the stratosphere or troposphere absorb incoming UV radiation © 2011 Pearson Education, Inc.

Global climate change occurs because increasing concentrations of greenhouse gases in a. b. c. d. e. The troposphere absorb outgoing IR radiation The stratosphere absorb outgoing IR radiation The troposphere absorb incoming UV radiation The stratosphere absorb incoming UV radiation Neither the stratosphere or troposphere absorb incoming UV radiation © 2011 Pearson Education, Inc.

Important factors that contribute to smog formation in the Las Angeles basin include which of the following? I. Ample summer sunshine II. Sea-level elevation III. High concentration of automobiles a. b. c. d. e. I only III only I and III only I, II, and III © 2011 Pearson Education, Inc.

Important factors that contribute to smog formation in the Los Angeles basin include which of the following? I. Ample summer sunshine II. Sea-level elevation III. High concentration of automobiles a. b. c. d. e. I only III only I and III only I, II, and III © 2011 Pearson Education, Inc.

Region where the protective ozone layer is located. a. b. c. d. e. Exosphere Mesosphere Stratosphere Thermosphere Troposphere © 2011 Pearson Education, Inc.

Region where the protective ozone layer is located. a. b. c. d. e. Exosphere Mesosphere Stratosphere Thermosphere Troposphere © 2011 Pearson Education, Inc.

Region that contains the majority of molecules in the atmosphere. a. b. c. d. e. Exosphere Mesosphere Stratosphere Thermosphere Troposphere © 2011 Pearson Education, Inc.

Region that contains the majority of molecules in the atmosphere. a. b. c. d. e. Exosphere Mesosphere Stratosphere Thermosphere Troposphere © 2011 Pearson Education, Inc.

Region largely responsible for the weather experienced at the Earth’s surface. a. b. c. d. e. Exosphere Mesosphere Stratosphere Thermosphere Troposphere © 2011 Pearson Education, Inc.

Region largely responsible for the weather experienced at the Earth’s surface. a. b. c. d. e. Exosphere Mesosphere Stratosphere Thermosphere Troposphere © 2011 Pearson Education, Inc.

Although levels of CFCs in the atmosphere are much lower than those of CO 2 , CFCs are still potent greenhouse gasses because they a. Remain in the atmosphere for only a brief time b. Lack natural sources c. Are much more efficient at absorbing thermal radiation d. Circulate through the troposphere more easily than CO 2 does e. Are more difficult to remove from smokestacks and tailpipes © 2011 Pearson Education, Inc.

Although levels of CFCs in the atmosphere are much lower than those of CO 2 , CFCs are still potent greenhouse gasses because they a. Remain in the atmosphere for only a brief time b. Lack natural sources c. Are much more efficient at absorbing thermal radiation d. Circulate through the troposphere more easily than CO 2 does e. Are more difficult to remove from smokestacks and tailpipes © 2011 Pearson Education, Inc.

Which of the following is an appropriate remediation strategy for removing radon gas from the home? a. b. c. d. e. Use filtered water for drinking and bathing. Do not occupy the basement of the home. Place monitors in suspect areas of the home. Remove and replace soil in crawl spaces under the home. Seal or ventilate places where radon enters the living space. © 2011 Pearson Education, Inc.

Which of the following is an appropriate remediation strategy for removing radon gas from the home? a. b. c. d. e. Use filtered water for drinking and bathing. Do not occupy the basement of the home. Place monitors in suspect areas of the home. Remove and replace soil in crawl spaces under the home. Seal or ventilate places where radon enters the living space. © 2011 Pearson Education, Inc.
Particulates can be removed from smokestack emissions by which of the following methods? a. b. c. d. e. Irradiation by UV light Electrostatic precipitators Catalytic converters Liquid chromatography Exhaust-stream aeration © 2011 Pearson Education, Inc.
Particulates can be removed from smokestack emissions by which of the following methods? a. b. c. d. e. Irradiation by UV light Electrostatic precipitators Catalytic converters Liquid chromatography Exhaust-stream aeration © 2011 Pearson Education, Inc.

QUESTION: Review The major component of Earth’s atmosphere is: a) Nitrogen gas b) Oxygen gas c) Argon gas d) Water vapor © 2011 Pearson Education, Inc.

QUESTION: Review The major component of Earth’s atmosphere is: a) Nitrogen gas b) Oxygen gas c) Argon gas d) Water vapor © 2011 Pearson Education, Inc.

QUESTION: Review _____ is defined as the ratio of water vapor in the atmosphere compared to the amount the atmosphere could contain. a) Atmospheric pressure b) Ozonification c) Temperature d) Relative humidity © 2011 Pearson Education, Inc.

QUESTION: Review _____ is defined as the ratio of water vapor in the atmosphere compared to the amount the atmosphere could contain. a) Atmospheric pressure b) Ozonification c) Temperature d) Relative humidity © 2011 Pearson Education, Inc.

QUESTION: Review If you were on a sailing ship going from Europe to the United States, you would want to be in the ____. a) Doldrums b) Trade winds c) Westerlies d) Polar cell © 2011 Pearson Education, Inc.

QUESTION: Review If you were on a sailing ship going from Europe to the United States, you would want to be in the ____. a) Doldrums b) Trade winds c) Westerlies d) Polar cell © 2011 Pearson Education, Inc.

QUESTION: Review The Clean Air Act does all of the following, EXCEPT: a) Allows higher levels of emissions of some criteria pollutants b) Provides funds for pollution control research c) Allows citizens to sue violators d) Sets standards for air quality © 2011 Pearson Education, Inc.

QUESTION: Review The Clean Air Act does all of the following, EXCEPT: a) Allows higher levels of emissions of some criteria pollutants b) Provides funds for pollution control research c) Allows citizens to sue violators d) Sets standards for air quality © 2011 Pearson Education, Inc.
QUESTION: Review Which criteria pollutant is colorless, odorless, and poses a risk to humans? a) Sulfur dioxide b) Nitrogen dioxide c) Tropospheric ozone d) Carbon monoxide © 2011 Pearson Education, Inc.
QUESTION: Review Which criteria pollutant is colorless, odorless, and poses a risk to humans? a) Sulfur dioxide b) Nitrogen dioxide c) Tropospheric ozone d) Carbon monoxide © 2011 Pearson Education, Inc.
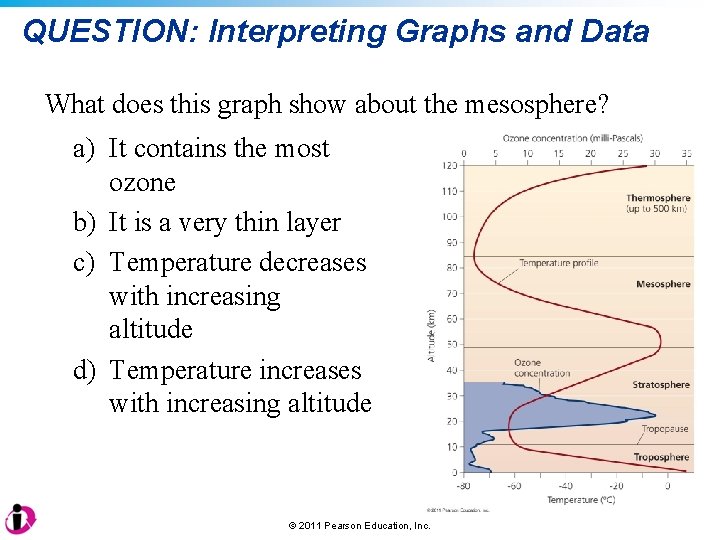
QUESTION: Interpreting Graphs and Data What does this graph show about the mesosphere? a) It contains the most ozone b) It is a very thin layer c) Temperature decreases with increasing altitude d) Temperature increases with increasing altitude © 2011 Pearson Education, Inc.
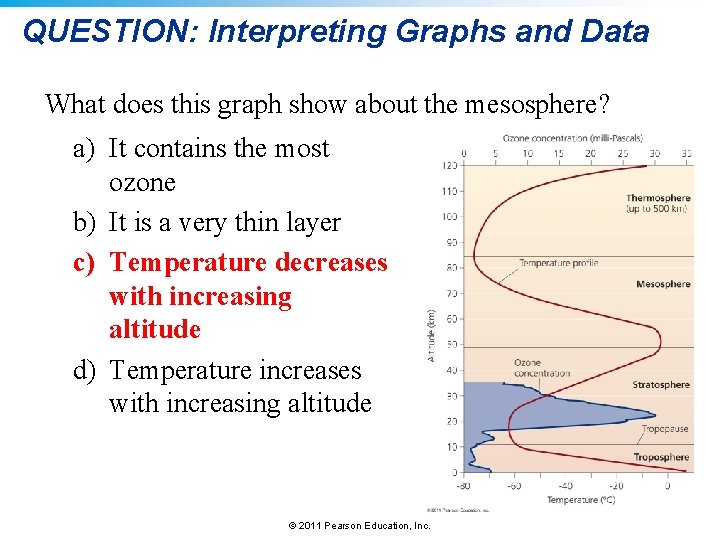
QUESTION: Interpreting Graphs and Data What does this graph show about the mesosphere? a) It contains the most ozone b) It is a very thin layer c) Temperature decreases with increasing altitude d) Temperature increases with increasing altitude © 2011 Pearson Education, Inc.
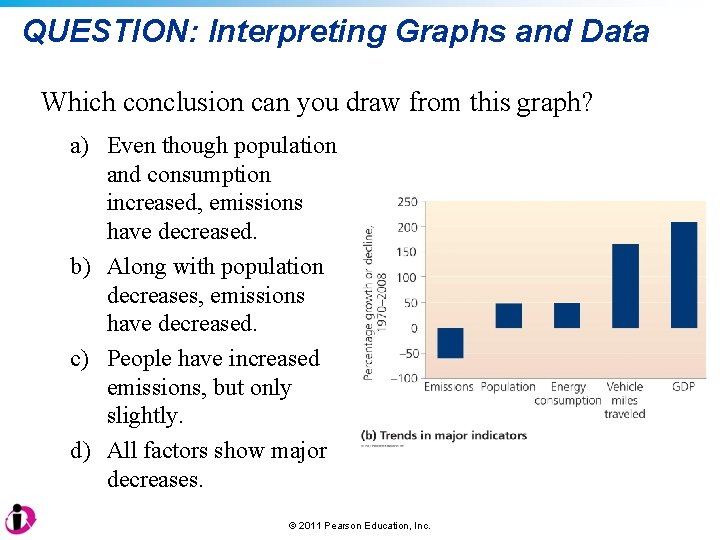
QUESTION: Interpreting Graphs and Data Which conclusion can you draw from this graph? a) Even though population and consumption increased, emissions have decreased. b) Along with population decreases, emissions have decreased. c) People have increased emissions, but only slightly. d) All factors show major decreases. © 2011 Pearson Education, Inc.
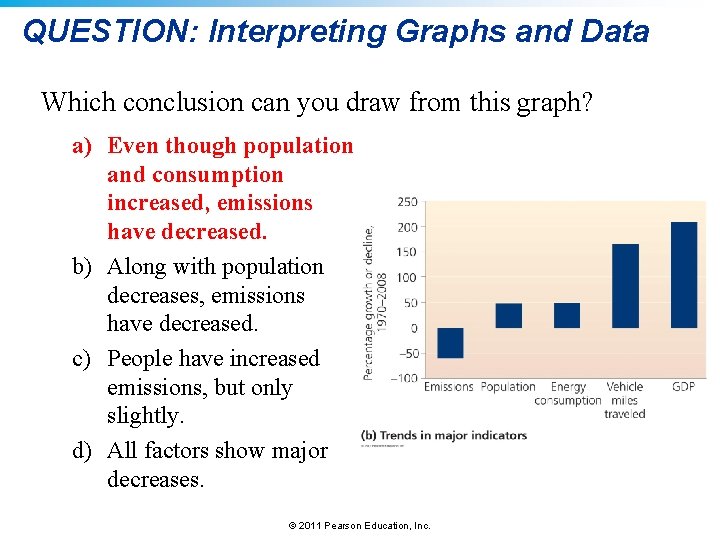
QUESTION: Interpreting Graphs and Data Which conclusion can you draw from this graph? a) Even though population and consumption increased, emissions have decreased. b) Along with population decreases, emissions have decreased. c) People have increased emissions, but only slightly. d) All factors show major decreases. © 2011 Pearson Education, Inc.
(A) H 2 O (B) CO 2 (C) CH 4 (D) O 3 (E) CCl 2 F 2 ___The most abundant nonanthropogenic gas ___ A greenhouse gas that is exclusively anthropogenic ___ A green house gas that, in the lower troposphere, is formed by photochemical reactions © 2011 Pearson Education, Inc.
(A) H 2 O (B) CO 2 (C) CH 4 (D) O 3 (E) CCl 2 F 2 A The most abundant nonanthropogenic gas E A greenhouse gas that is exclusively anthropogenic D A green house gas that, in the lower troposphere, is formed by photochemical reactions © 2011 Pearson Education, Inc.
- Slides: 65