Lecture Outline Sulfuric Acid History of Manufacture Development






- Slides: 19

Lecture Outline - Sulfuric Acid - History of Manufacture Development - Manufacture - Oleum Production - Heat Integration Issues / By-products - Markets - Usage in Caprolactam Manufacture

History of Manufacture of Sulfuric Acid • One of the oldest industrially applied processes. Discovered by a Persian alchemist in the tenth century. • Saltpeter and sulfur were mixed in a glass container and burned in a moist atmosphere. Acid was collected from the condensed vapors. • In England, 1746, the lead chamber reactor was invented. This invention allowed for higher production rates (<78%). • In England, 1831, a patent was filed that described the oxidation of sulfur dioxide over a platinum catalyst, the Contact Process. This new process increased yields of reaction from 70 to above 95%. • In 1913, BASF was granted a patent for the use of vanadium pentoxide as a catalyst for the Contact Process • By the 1930’s, vanadium pentoxide was becoming the dominate catalyst used because of insensitivities to poisons and lower cost. • In 1960 a patent application was filed by Bayer using the so called double-catalyst process (double absorption).

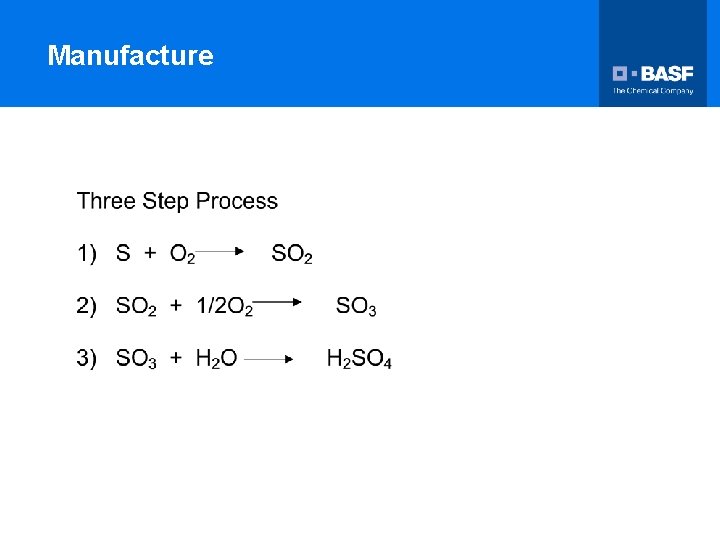
Manufacture

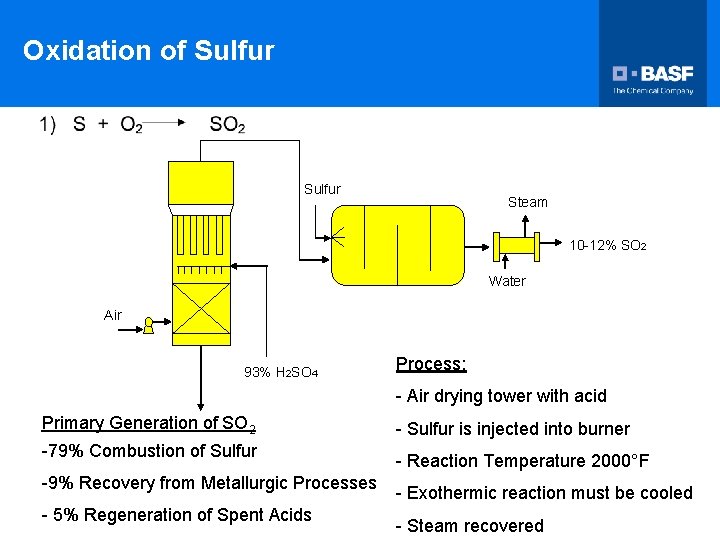
Oxidation of Sulfur Steam 10 -12% SO 2 Water Air 93% H 2 SO 4 Process: - Air drying tower with acid Primary Generation of SO 2 -79% Combustion of Sulfur -9% Recovery from Metallurgic Processes - 5% Regeneration of Spent Acids - Sulfur is injected into burner - Reaction Temperature 2000°F - Exothermic reaction must be cooled - Steam recovered

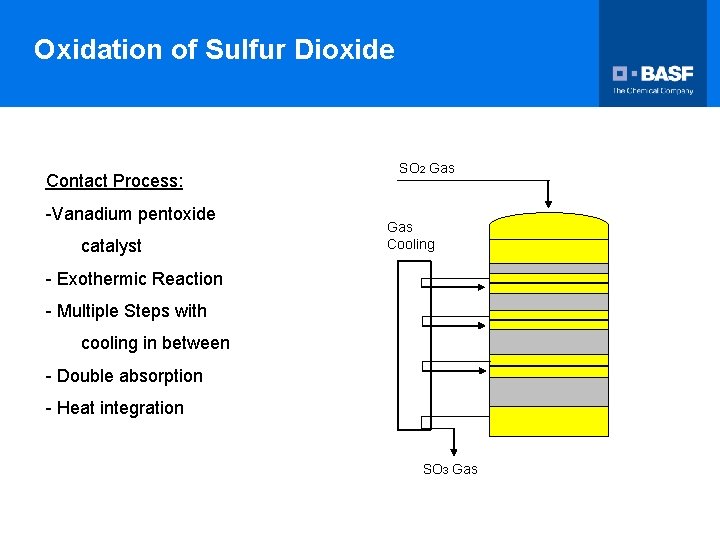
Oxidation of Sulfur Dioxide Contact Process: -Vanadium pentoxide catalyst SO 2 Gas Cooling - Exothermic Reaction - Multiple Steps with cooling in between - Double absorption - Heat integration SO 3 Gas

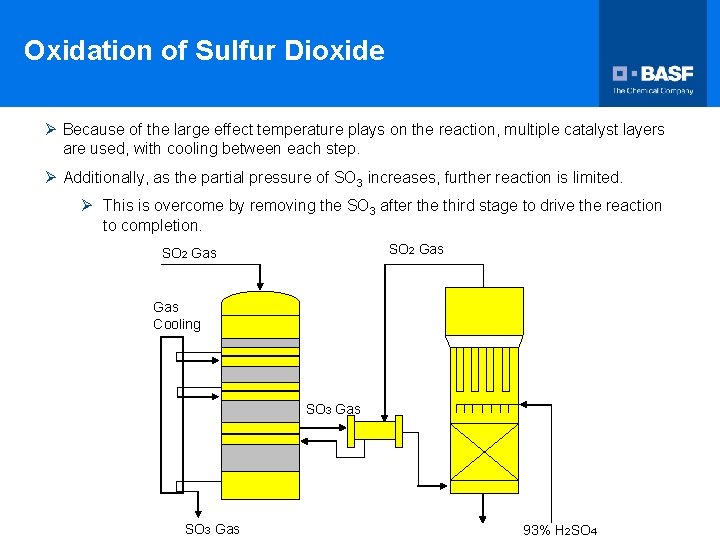
Oxidation of Sulfur Dioxide Ø Because of the large effect temperature plays on the reaction, multiple catalyst layers are used, with cooling between each step. Ø Additionally, as the partial pressure of SO 3 increases, further reaction is limited. Ø This is overcome by removing the SO 3 after the third stage to drive the reaction to completion. SO 2 Gas Gas Cooling SO 3 Gas 93% H 2 SO 4

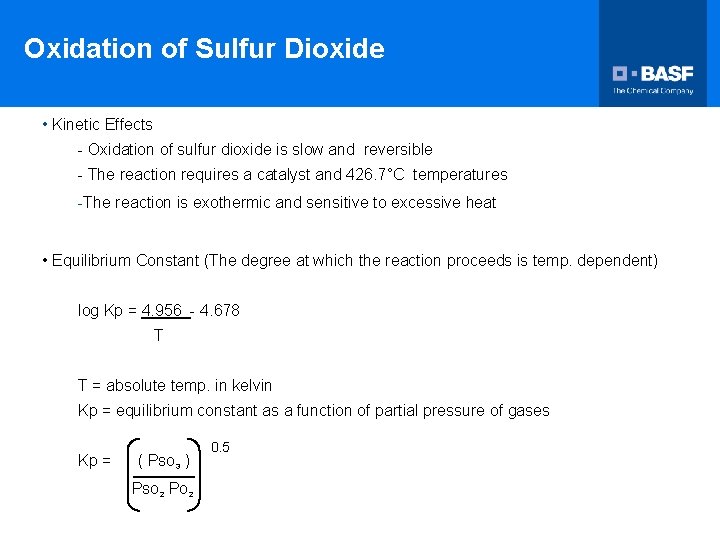
Oxidation of Sulfur Dioxide • Kinetic Effects - Oxidation of sulfur dioxide is slow and reversible - The reaction requires a catalyst and 426. 7°C temperatures -The reaction is exothermic and sensitive to excessive heat • Equilibrium Constant (The degree at which the reaction proceeds is temp. dependent) log Kp = 4. 956 - 4. 678 T T = absolute temp. in kelvin Kp = equilibrium constant as a function of partial pressure of gases Kp = ( PSO 3 ) PSO 2 PO 2 0. 5

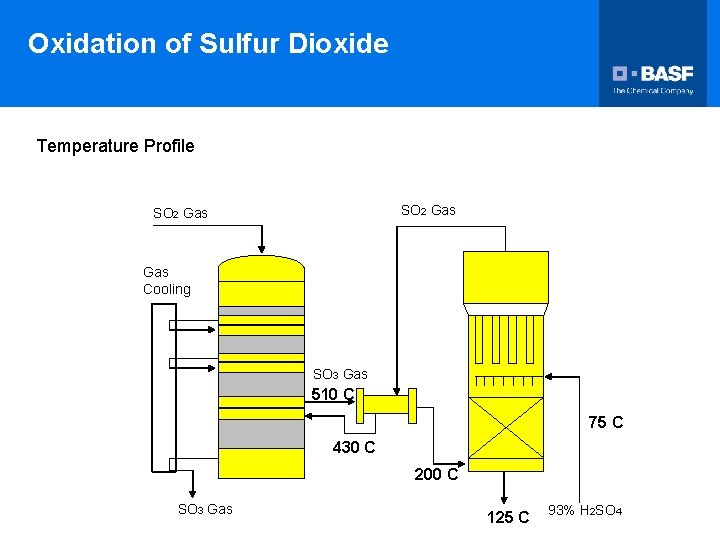
Oxidation of Sulfur Dioxide Temperature Profile SO 2 Gas Gas Cooling SO 3 Gas 510 C 75 C 430 C 200 C SO 3 Gas 125 C 93% H 2 SO 4

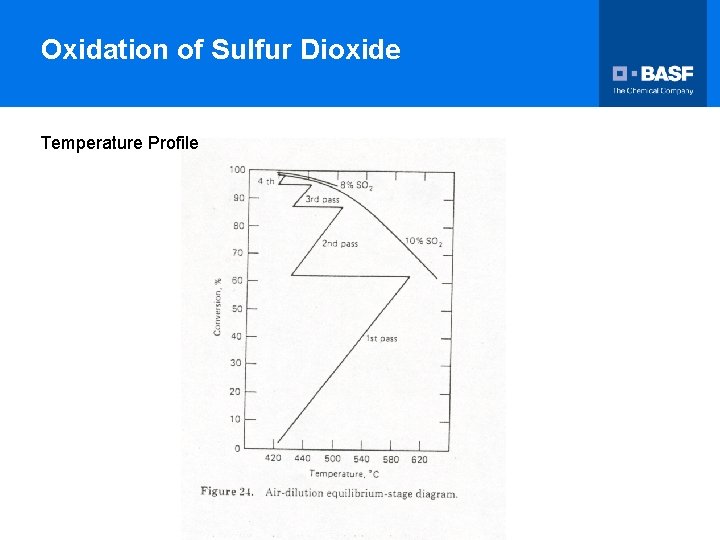
Oxidation of Sulfur Dioxide Temperature Profile

Oxidation of Sulfur Dioxide Typical Catalyst Distribution

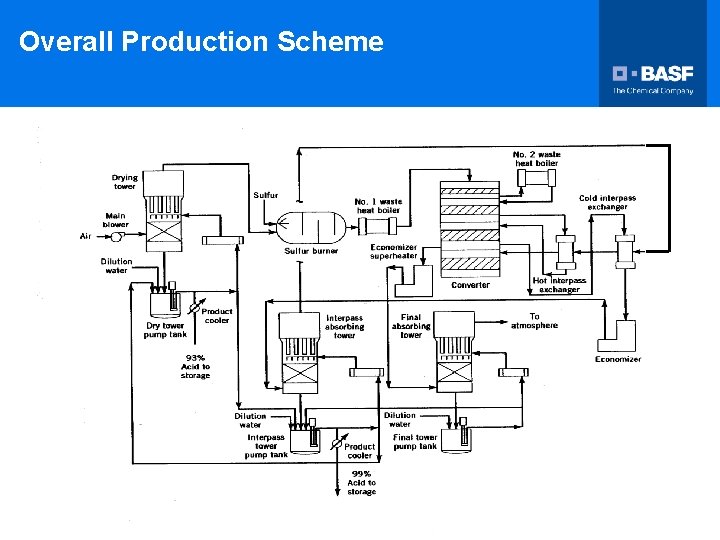
Overall Production Scheme

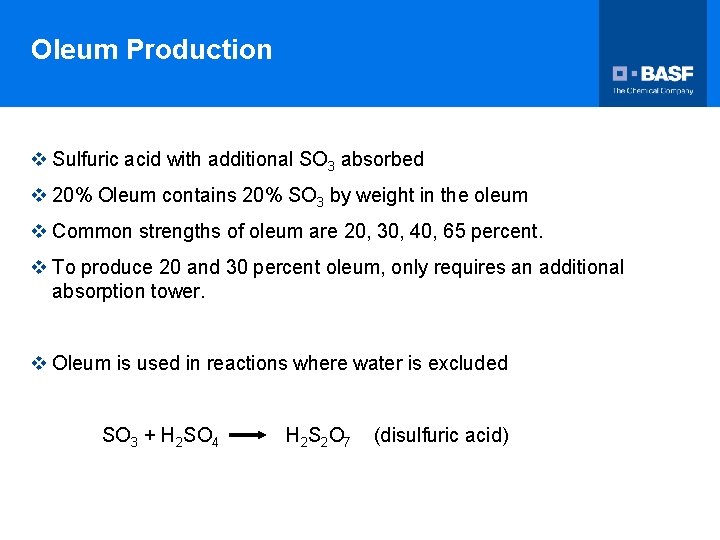
Oleum Production v Sulfuric acid with additional SO 3 absorbed v 20% Oleum contains 20% SO 3 by weight in the oleum v Common strengths of oleum are 20, 30, 40, 65 percent. v To produce 20 and 30 percent oleum, only requires an additional absorption tower. v Oleum is used in reactions where water is excluded SO 3 + H 2 SO 4 H 2 S 2 O 7 (disulfuric acid)

Reaction By-products / Heat Integration By-products v 57 to 64% of the energy input generates steam v Steam energy is used to drive the turbine that supplies power to the main air blower v Additional steam remaining is tolled internally for other plant operations v SO 2/SO 3 is vented in small amounts and is federally regulated. Heat Integration v Steam is used to pre-heat and vapor from the absorption towers used to cool v Minimizes the cost of manufacturing to maximize the profit.

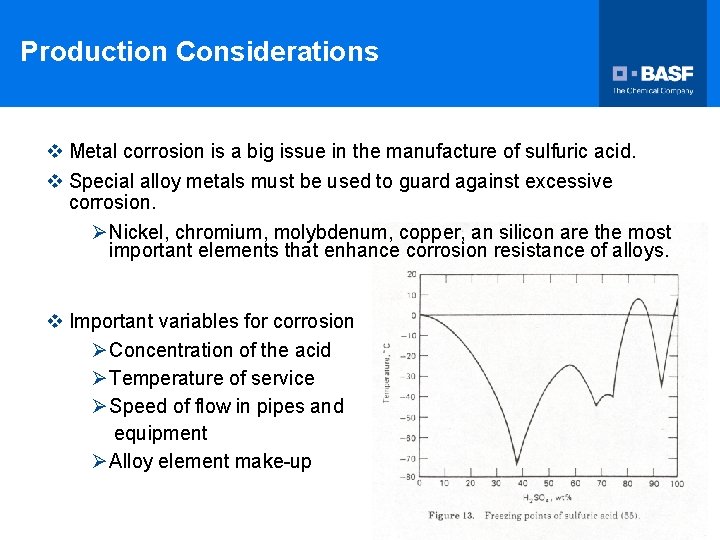
Production Considerations v Metal corrosion is a big issue in the manufacture of sulfuric acid. v Special alloy metals must be used to guard against excessive corrosion. Ø Nickel, chromium, molybdenum, copper, an silicon are the most important elements that enhance corrosion resistance of alloys. v Important variables for corrosion Ø Concentration of the acid Ø Temperature of service Ø Speed of flow in pipes and equipment Ø Alloy element make-up

World Production of Sulfuric Acid

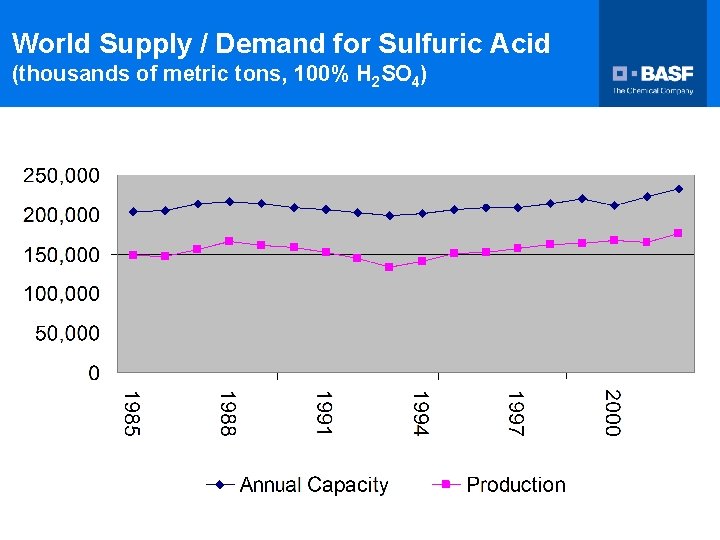
World Supply / Demand for Sulfuric Acid (thousands of metric tons, 100% H 2 SO 4)

Markets for Sulfuric Acid v The fertilizer market is the largest U. S. single use for sulfuric acid and consumes 50 -65 percent of all produced. v Second is the organic chemical industry. Production of plastics and synthetic fibers are examples. v Production of Ti. O 2 consumes large quantities of sulfuric acid. Ti. O 2 is a white pigment used in paints and plastics. v In the metal industry, sulfuric acid is used for pickling ferrous and nonferrous materials and in the recovery of copper, nickel, and zinc from low-grade ores. v Finally, the petroleum industry uses acid as a catalyst for various reactions.

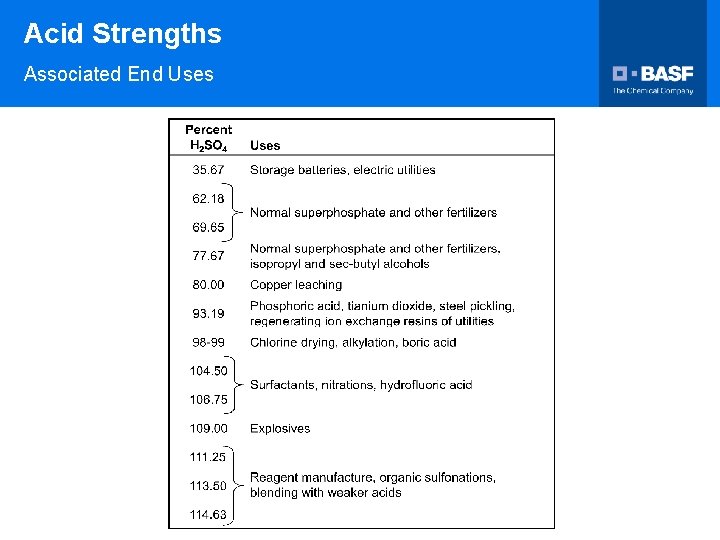
Acid Strengths Associated End Uses

Usage in Caprolactam Manufacture Production and consumption figures for caprolactam manufacture