Lecture Objectives Learn about Desiccant systems Start energy

Lecture Objectives: • Learn about - Desiccant systems • Start energy production systems - Sorption cooling

Desiccant Systems: Reading assignment • Desiccant systems • http: //www. ce. utexas. edu/prof/Novoselac/classes/ CE 397 b/Handouts/1 -s 2. 0 -S 1364032109001737 main. pdf

Desiccant wheel
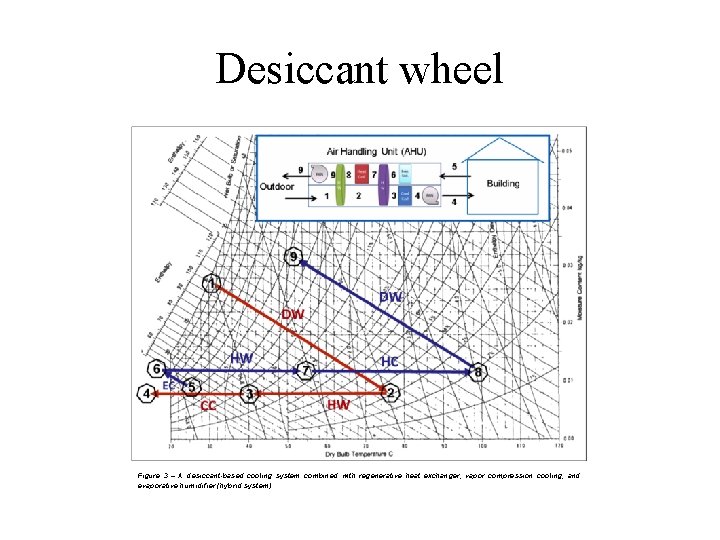
Desiccant wheel Figure 3 – A desiccant-based cooling system combined with regenerative heat exchanger, vapor compression cooling, and evaporative humidifier (hybrid system).
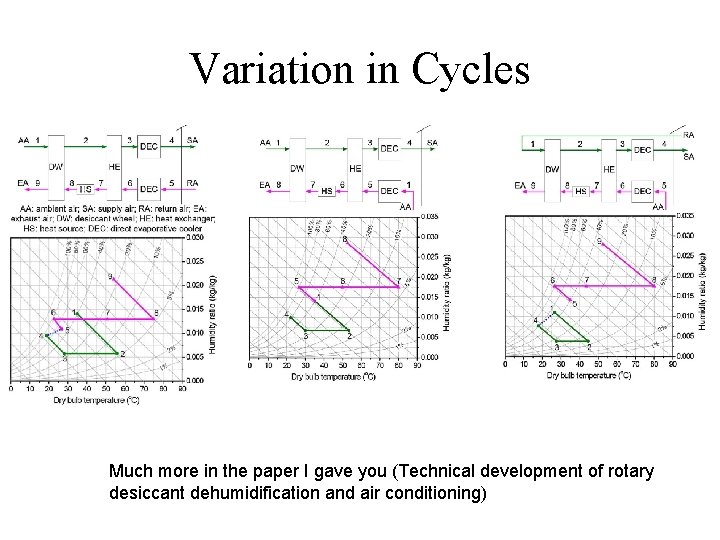
Variation in Cycles Much more in the paper I gave you (Technical development of rotary desiccant dehumidification and air conditioning)
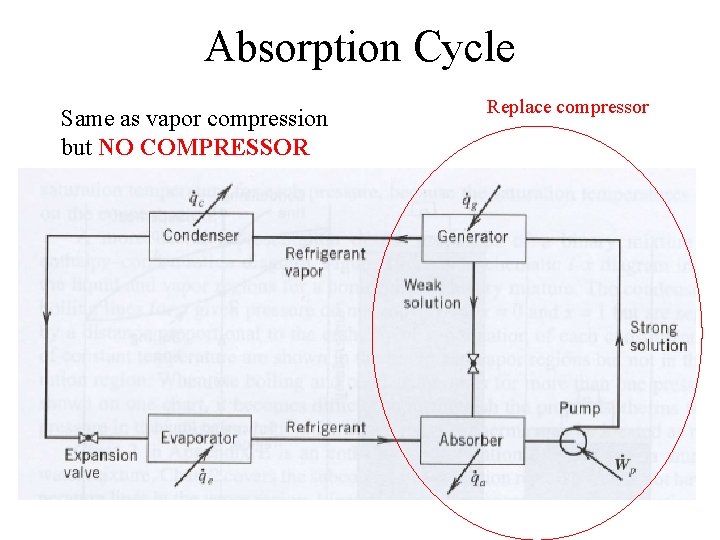
Absorption Cycle Same as vapor compression but NO COMPRESSOR Replace compressor
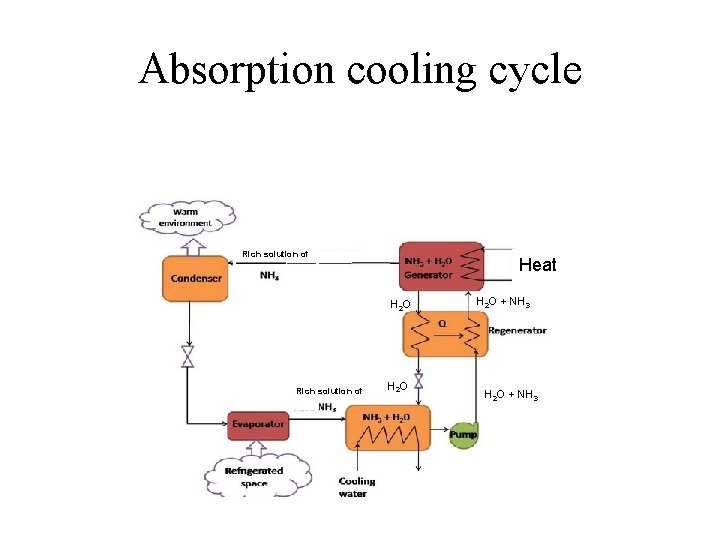
Absorption cooling cycle Rich solution of Heat H 2 O Rich solution of H 2 O + NH 3
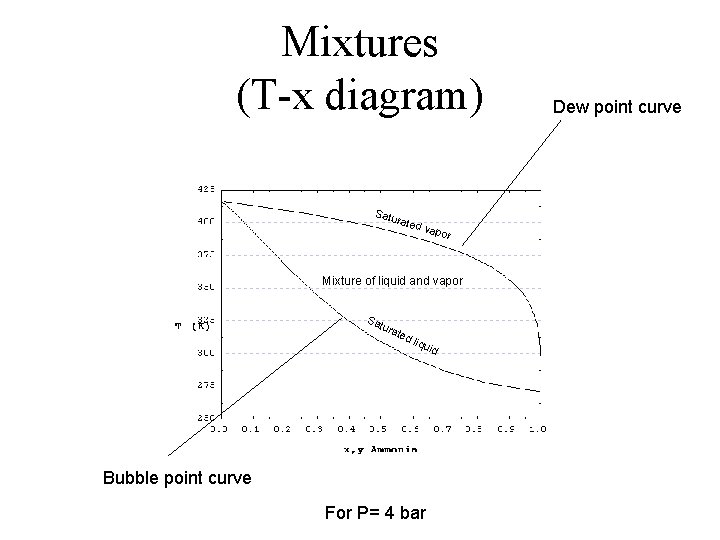
Mixtures (T-x diagram) Satu rate d va por Mixture of liquid and vapor Sa tura ted liqu id Bubble point curve For P= 4 bar Dew point curve

h-x diagram hfg for H 2 O hfg for NH 3 Isotherms are shown only in liquid region
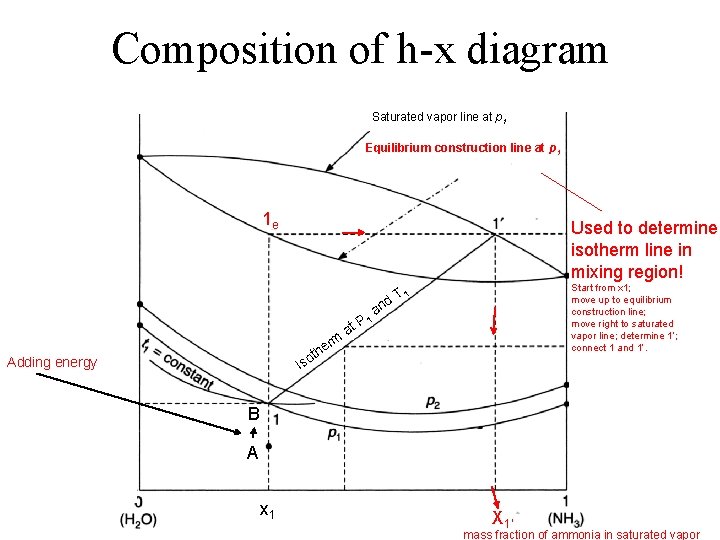
Composition of h-x diagram Saturated vapor line at p 1 Equilibrium construction line at p 1 1 e Used to determine isotherm line in mixing region! Start from x 1; move up to equilibrium construction line; move right to saturated vapor line; determine 1’; connect 1 and 1’. 1 d. T rm at P 1 an e oth Adding energy Is B A x 1 X 1’ mass fraction of ammonia in saturated vapor
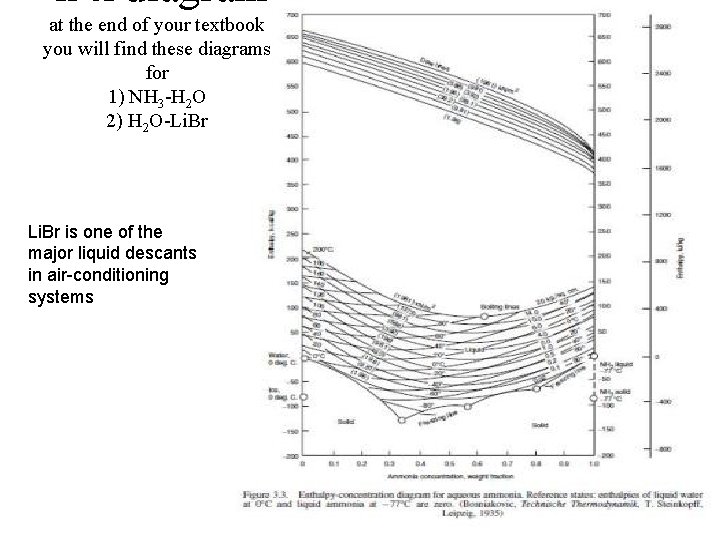
h-x diagram at the end of your textbook you will find these diagrams for 1) NH 3 -H 2 O 2) H 2 O-Li. Br is one of the major liquid descants in air-conditioning systems
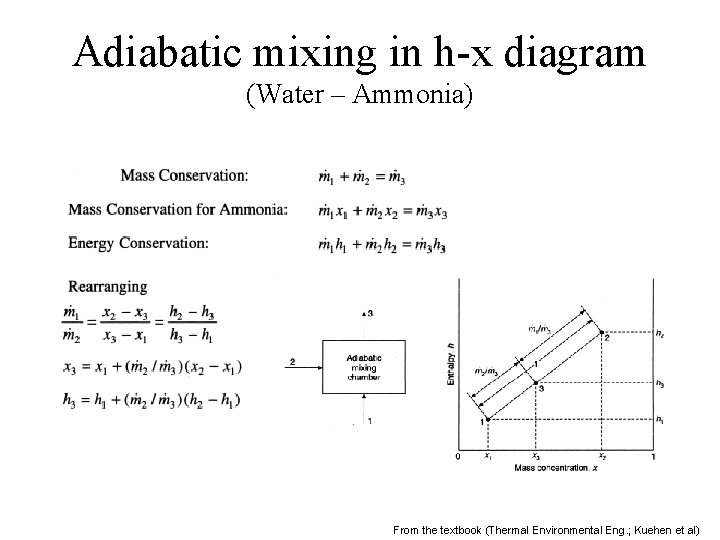
Adiabatic mixing in h-x diagram (Water – Ammonia) From the textbook (Thermal Environmental Eng. ; Kuehen et al)
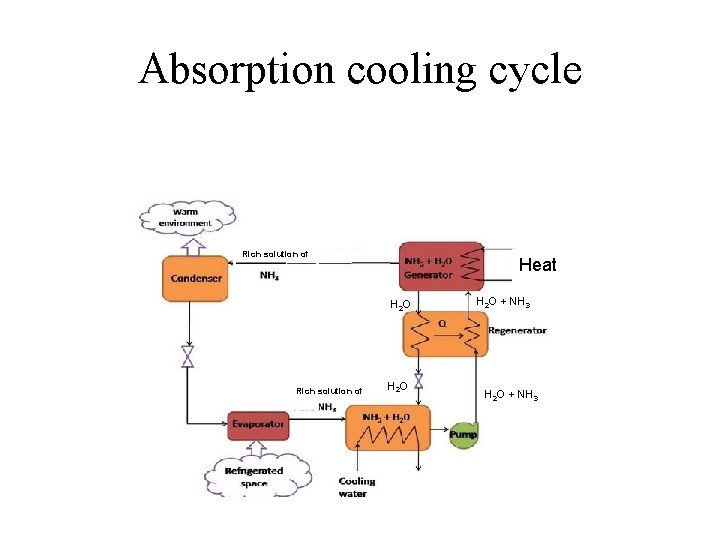
Absorption cooling cycle Rich solution of Heat H 2 O Rich solution of H 2 O + NH 3
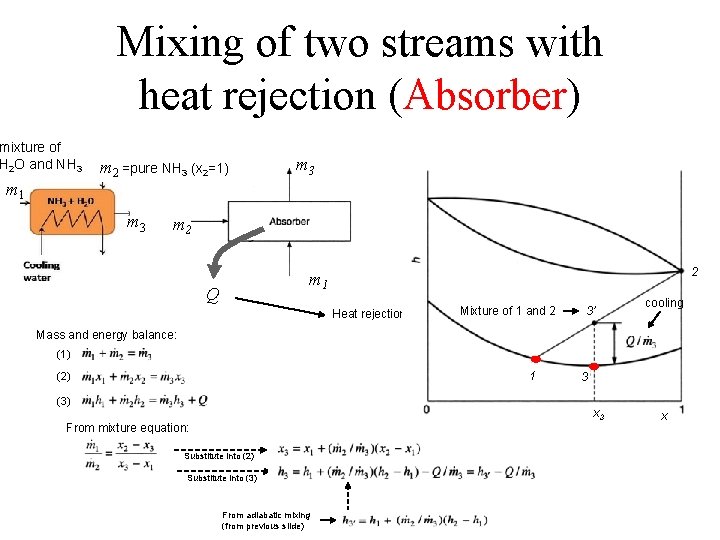
Mixing of two streams with heat rejection (Absorber) mixture of H 2 O and NH 3 m 1 m 2 =pure NH 3 (x 2=1) m 3 m 2 2 m 1 Q Heat rejection Mixture of 1 and 2 3’ cooling Mass and energy balance: (1) 1 (2) (3) 3 x 3 From mixture equation: Substitute into (2) Substitute into (3) From adiabatic mixing (from previous slide) x
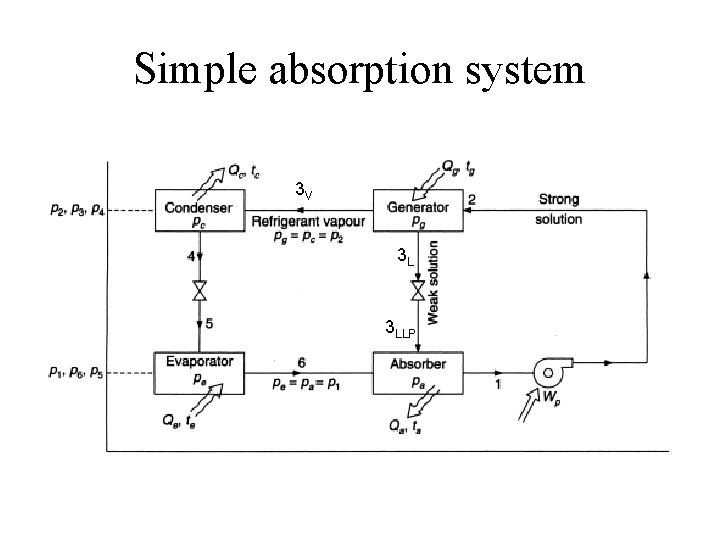
Simple absorption system 3 V 3 L 3 LLP
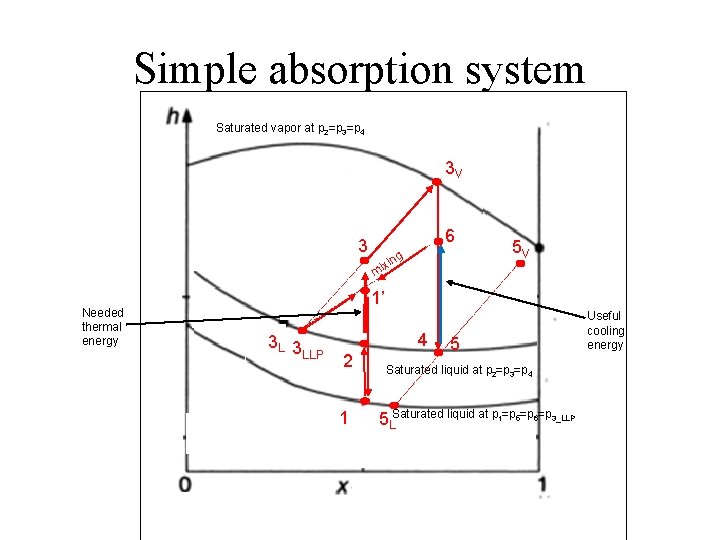
Simple absorption system Saturated vapor at p 2=p 3=p 4 3 V 3 Needed thermal energy 6 g xin i m 5 V 1’ 3 L 3 LLP 4 2 1 5 Saturated liquid at p 2=p 3=p 4 5 LSaturated liquid at p 1=p 5=p 6=p 3_LLP Useful cooling energy
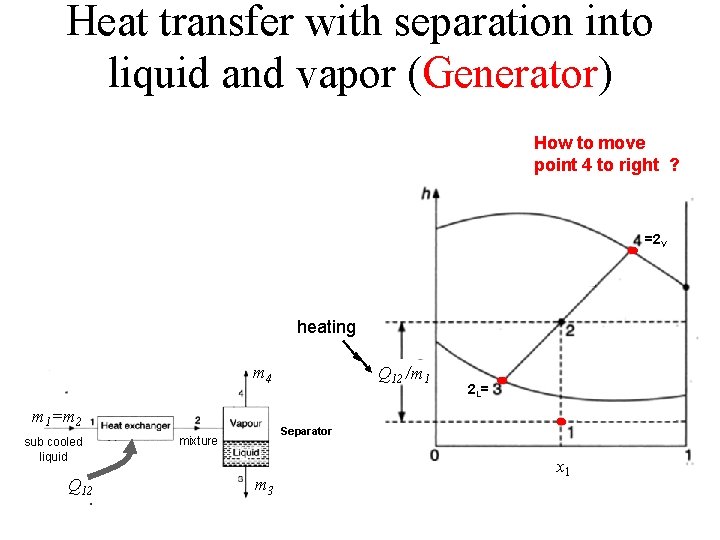
Heat transfer with separation into liquid and vapor (Generator) How to move point 4 to right ? =2 V heating m 4 =m 2 m 1=m 2 sub cooled liquid Q 12 mixture Q 12 /m 1 2 L= Separator m 3 x 1
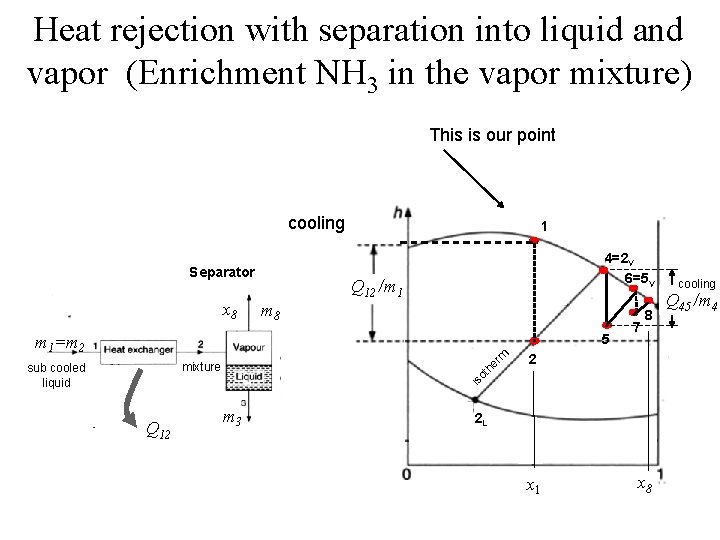
Heat rejection with separation into liquid and vapor (Enrichment NH 3 in the vapor mixture) This is our point cooling Separator x 8 1 4=2 V 6=5 V Q 12 /m 1 m 8 5 rm m 1=m 2 2 is o th e mixture sub cooled liquid 7 8 Q 12 m 3 2 L x 1 x 8 cooling Q 45 /m 4
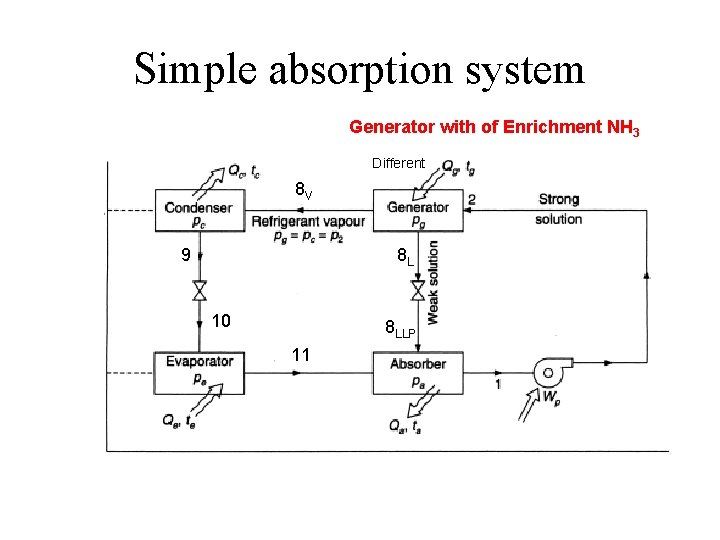
Simple absorption system Generator with of Enrichment NH 3 Different 8 V 9 8 L 10 8 LLP 11
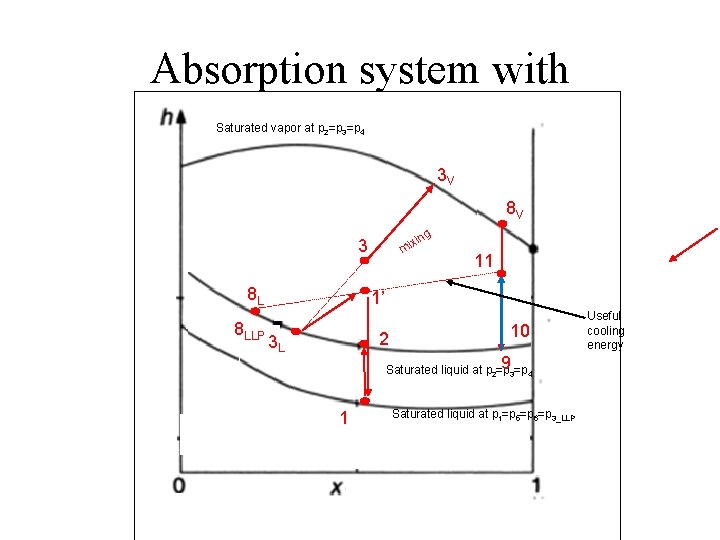
Absorption system with Saturated vapor at p 2=p 3=p 4 3 V 8 V ng xi mi 3 8 L 8 LLP 11 1’ 2 3 L 10 93=p 4 Saturated liquid at p 2=p 1 Saturated liquid at p 1=p 5=p 6=p 3_LLP Useful cooling energy
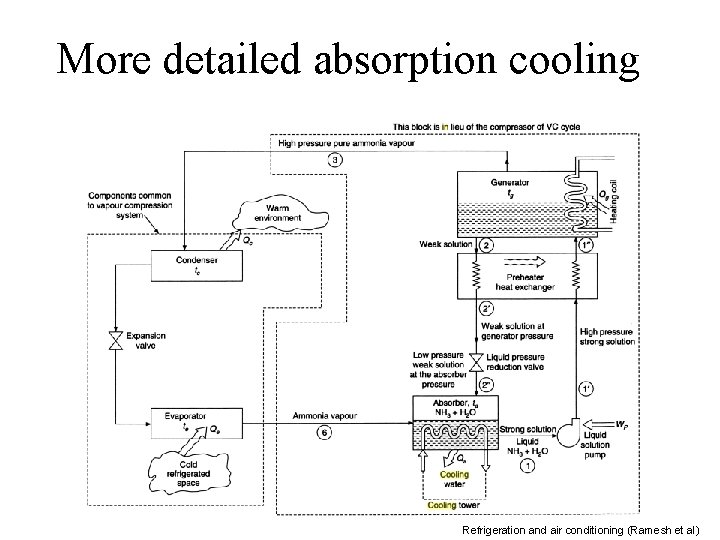
More detailed absorption cooling Refrigeration and air conditioning (Ramesh et al)
- Slides: 21