Lecture Objectives Aabsorption cooling cycles Absorption cooling cycle

Lecture Objectives: • Aabsorption cooling cycles
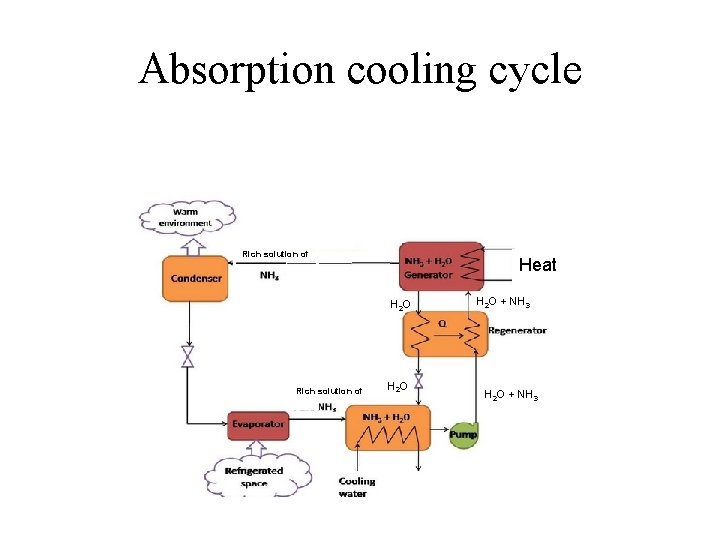
Absorption cooling cycle Rich solution of Heat H 2 O Rich solution of H 2 O + NH 3

Simple absorption system 3 V 3 L 3 LLP

Simple absorption system Saturated vapor at p 2=p 3=p 4 3 V 3 Needed thermal energy 6 g xin i m 5 V 1’ 3 L 3 LLP 4 2 1 5 Saturated liquid at p 2=p 3=p 4 5 LSaturated liquid at p 1=p 5=p 6=p 3_LLP Useful cooling energy
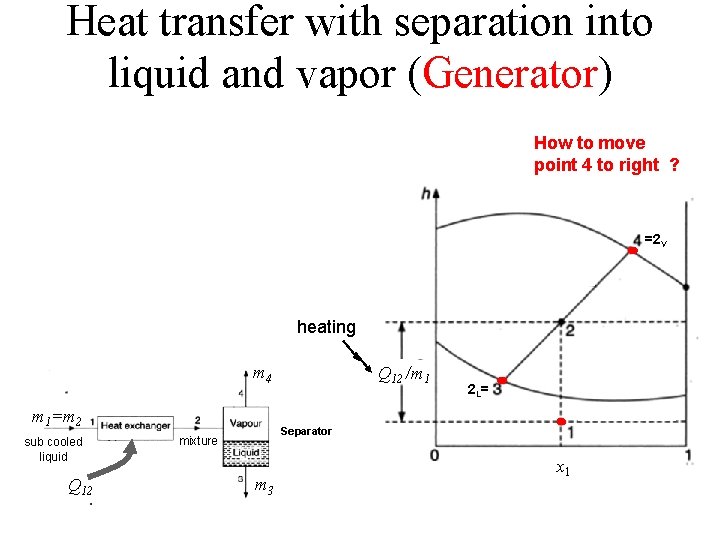
Heat transfer with separation into liquid and vapor (Generator) How to move point 4 to right ? =2 V heating m 4 =m 2 m 1=m 2 sub cooled liquid Q 12 mixture Q 12 /m 1 2 L= Separator m 3 x 1
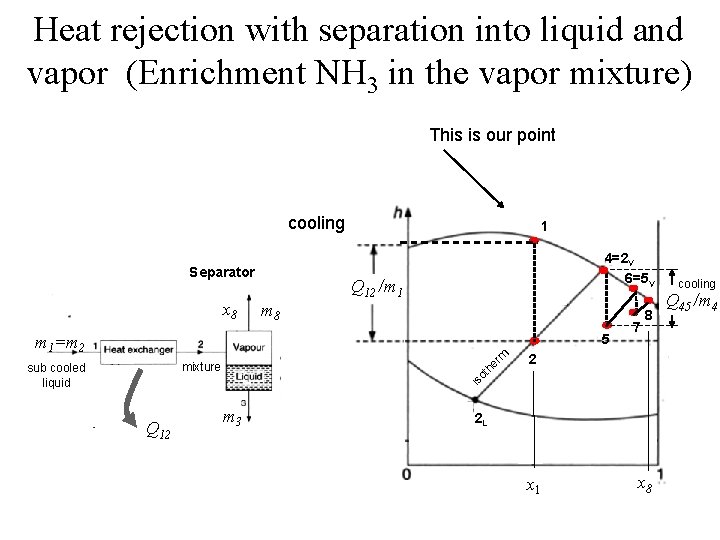
Heat rejection with separation into liquid and vapor (Enrichment NH 3 in the vapor mixture) This is our point cooling Separator x 8 1 4=2 V 6=5 V Q 12 /m 1 m 8 5 rm m 1=m 2 2 is o th e mixture sub cooled liquid 7 8 Q 12 m 3 2 L x 1 x 8 cooling Q 45 /m 4

Simple absorption system Generator with of Enrichment NH 3 Different 8 V 9 8 L 10 8 LLP 11
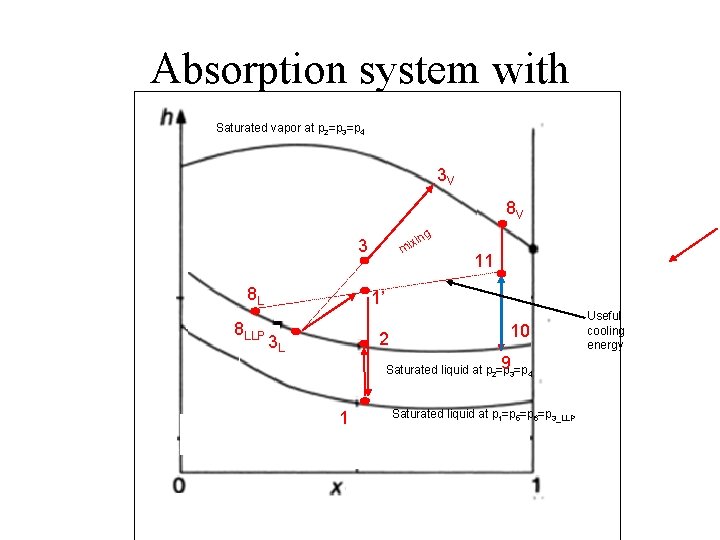
Absorption system with Saturated vapor at p 2=p 3=p 4 3 V 8 V ng xi mi 3 8 L 8 LLP 11 1’ 2 3 L 10 93=p 4 Saturated liquid at p 2=p 1 Saturated liquid at p 1=p 5=p 6=p 3_LLP Useful cooling energy
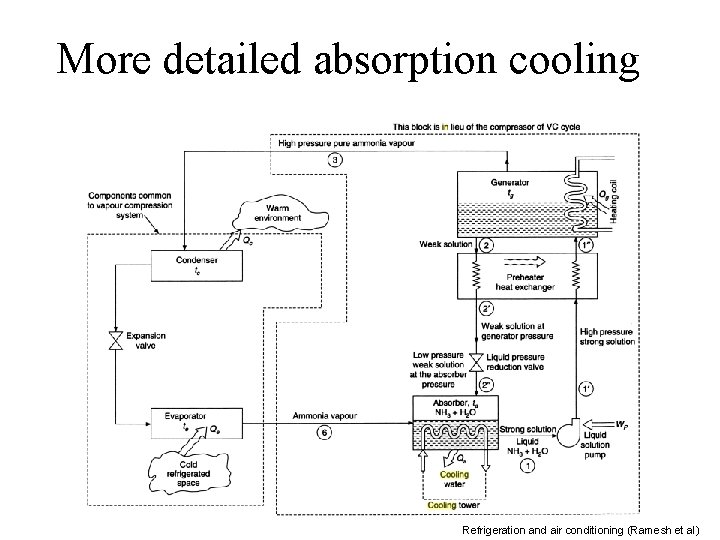
More detailed absorption cooling Refrigeration and air conditioning (Ramesh et al)
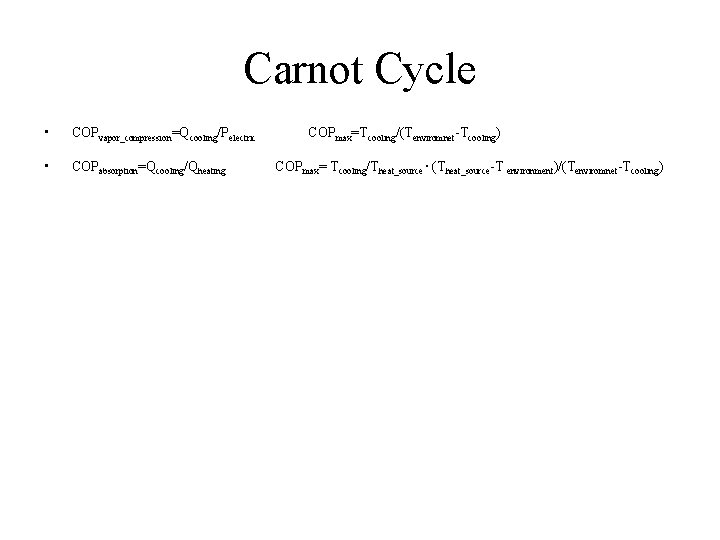
Carnot Cycle • COPvapor_compression=Qcooling/Pelectric • COPabsorption=Qcooling/Qheating COPmax=Tcooling/(Tenviromnet-Tcooling) COPmax= Tcooling/Theat_source ∙ (Theat_source-T environment)/(Tenviromnet-Tcooling)
- Slides: 10