Lecture No Two Examples Problems Work done Internal

Lecture No Two Examples & Problems Work done, Internal energy and heat “thermal” energy for various processes 1
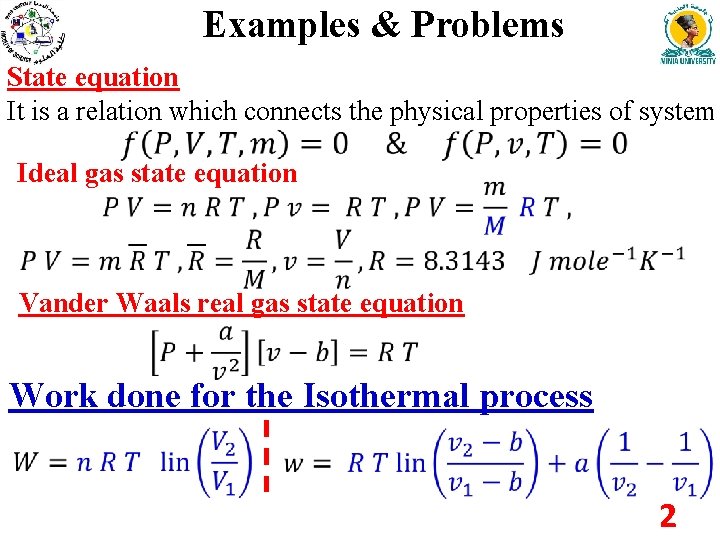
Examples & Problems State equation It is a relation which connects the physical properties of system Ideal gas state equation Vander Waals real gas state equation Work done for the Isothermal process 2
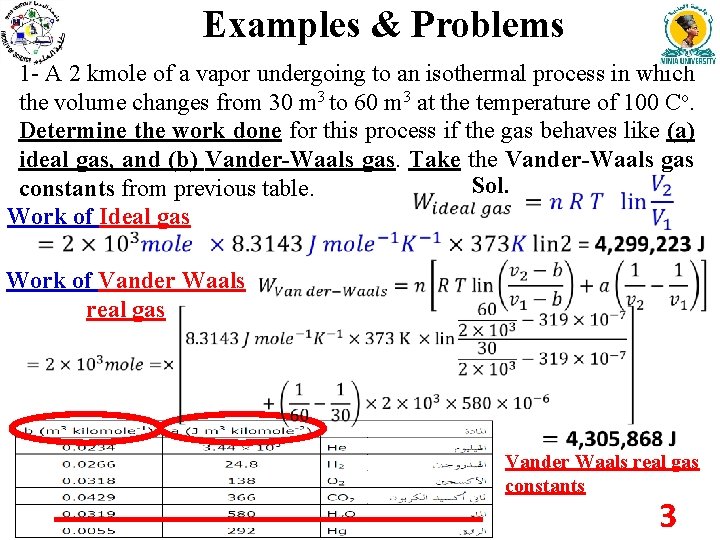
Examples & Problems 1 - A 2 kmole of a vapor undergoing to an isothermal process in which the volume changes from 30 m 3 to 60 m 3 at the temperature of 100 Co. Determine the work done for this process if the gas behaves like (a) ideal gas, and (b) Vander-Waals gas. Take the Vander-Waals gas Sol. constants from previous table. Work of Ideal gas Work of Vander Waals real gas constants 3
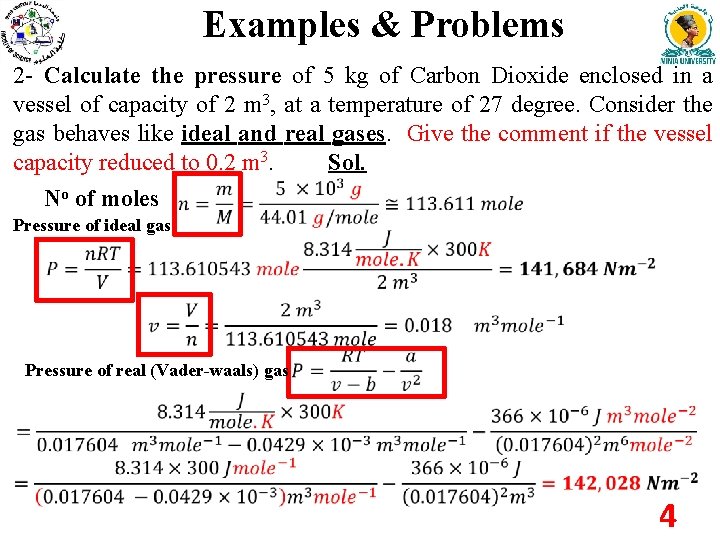
Examples & Problems 2 - Calculate the pressure of 5 kg of Carbon Dioxide enclosed in a vessel of capacity of 2 m 3, at a temperature of 27 degree. Consider the gas behaves like ideal and real gases. Give the comment if the vessel capacity reduced to 0. 2 m 3. Sol. No of moles Pressure of ideal gas Pressure of real (Vader-waals) gas 4

Examples & Problems 5
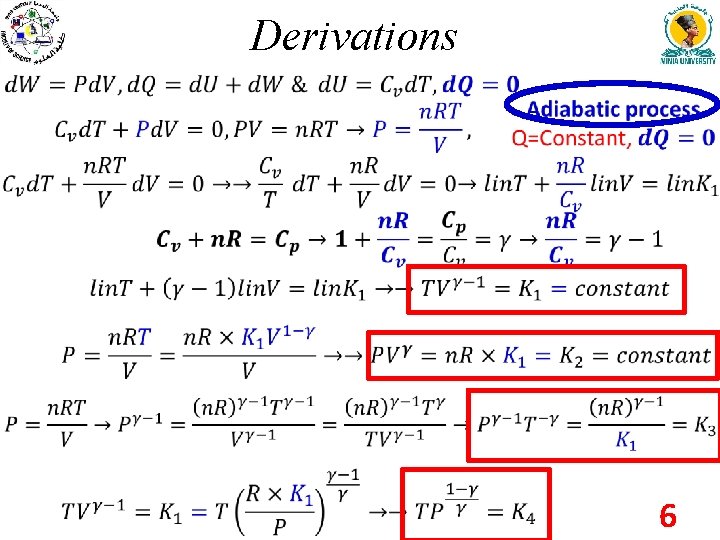
Derivations 6
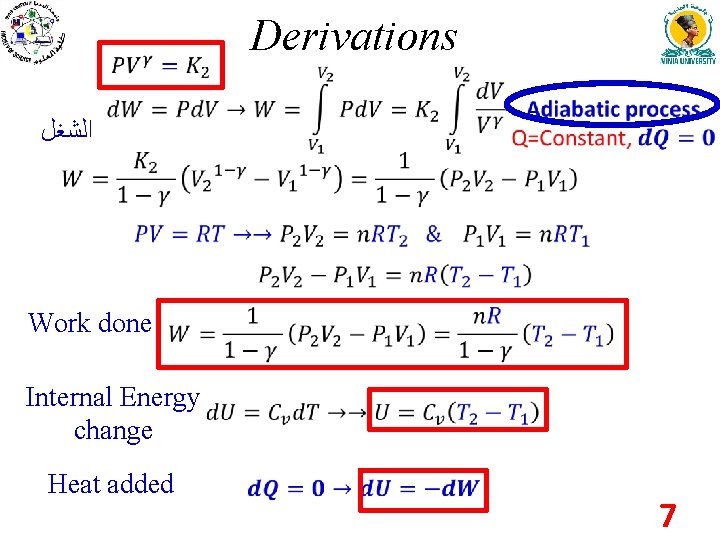
Derivations ﺍﻟﺸﻐﻞ Work done Internal Energy change Heat added 7
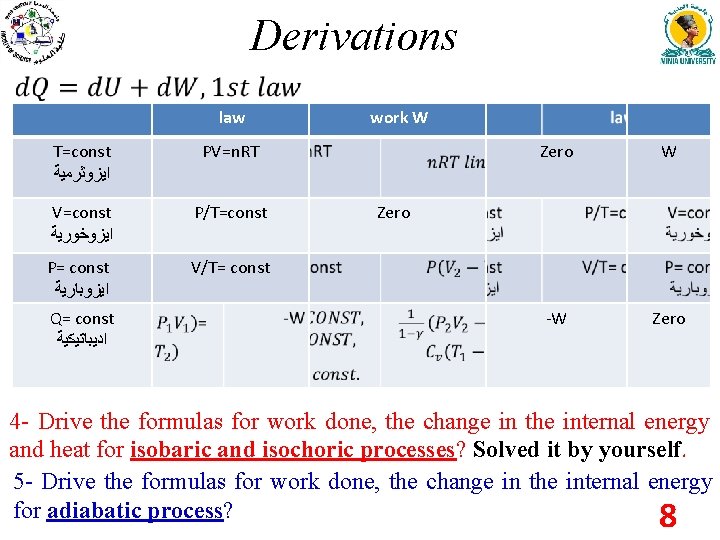
Derivations law T=const ﺍﻳﺰﻭﺛﺮﻣﻴﺔ PV=n. RT V=const ﺍﻳﺰﻭﺧﻮﺭﻳﺔ P/T=const P= const ﺍﻳﺰﻭﺑﺎﺭﻳﺔ V/T= const Q= const ﺍﺩﻳﺒﺎﺗﻴﻜﻴﺔ work W Zero W -W Zero 4 - Drive the formulas for work done, the change in the internal energy and heat for isobaric and isochoric processes? Solved it by yourself. 5 - Drive the formulas for work done, the change in the internal energy for adiabatic process? 8
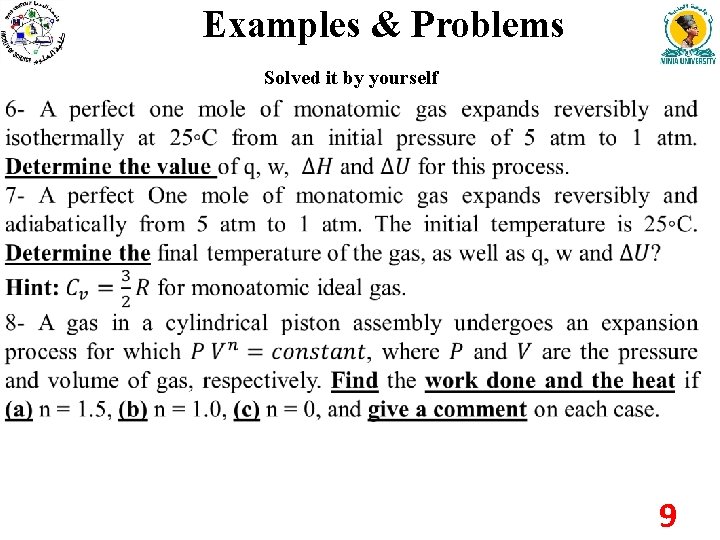
Examples & Problems Solved it by yourself 9

Lecture No Two End of Lecture With best wishes for all students 10
- Slides: 10