Lecture No 5 Diagnosis and Management of IEM








- Slides: 26

Lecture No. 5 Diagnosis and Management of IEM Dr. Obeid Shanab. Assistant professor of Biochemistry, Clinical Biochemistry and Cancer Biology

Diagnosis and Management There are 3 important steps in the diagnosis and management of IEM: 1. Suspicion 2. Evaluation 3. Treatment

Suspicion • An important key to diagnosing IEM is thinking about the possibility in the first place • The symptoms are very common and non-specific • Screening allows for the differential diagnosis

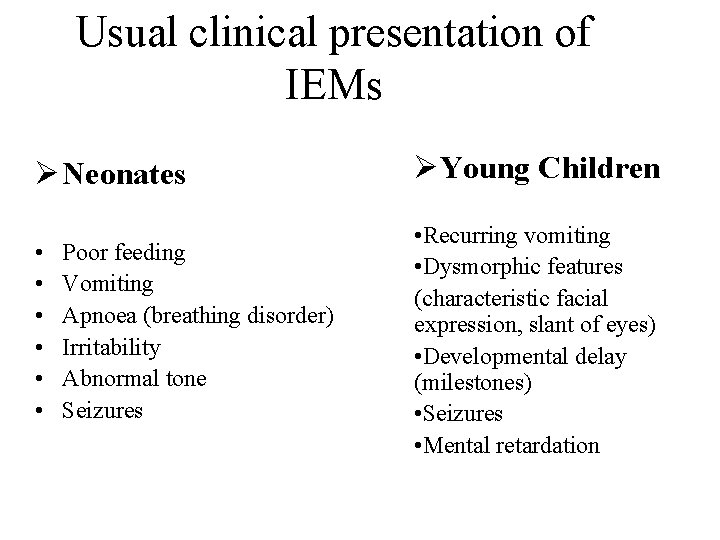
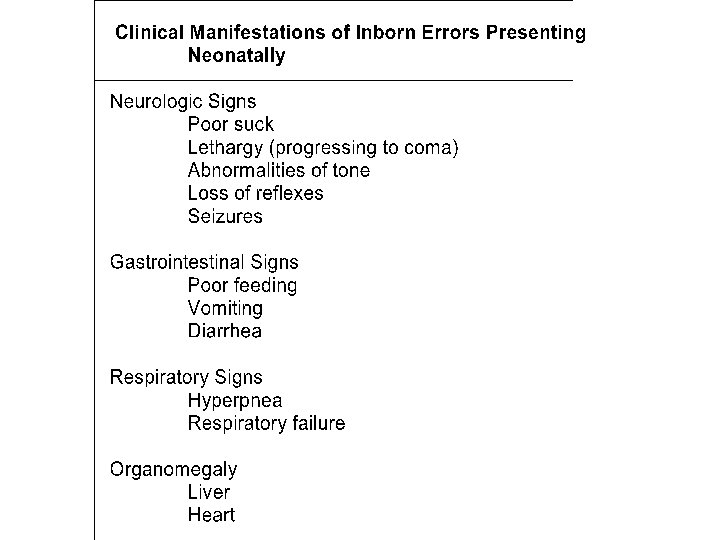
Usual clinical presentation of IEMs Ø Neonates ØYoung Children • • Recurring vomiting • Dysmorphic features (characteristic facial expression, slant of eyes) • Developmental delay (milestones) • Seizures • Mental retardation Poor feeding Vomiting Apnoea (breathing disorder) Irritability Abnormal tone Seizures


Developmental delay

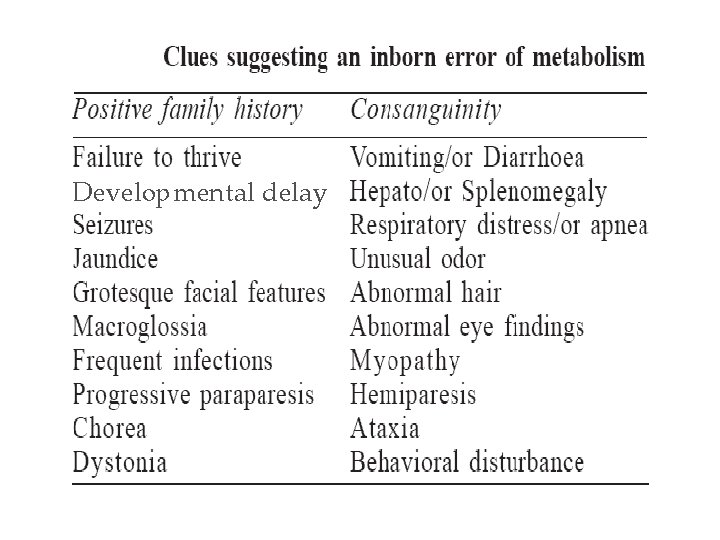
Evaluation-1 Once the possibility of an IEM is suspected, how should it be evaluated? • History Ø An important clue is a history of deterioration after an initial period of good health Ø Developmental delay Ø Change in diet and unusual dietary preferences Family history Ø Most IEMs are autosomal recessive: any other siblings with the same condition? Ø Consanguineous marriages increases the incidence of recessive disease

Evaluation-2 • There are two different types of testing for metabolic conditions: screening tests and disease-specific diagnostic testing • Initial screening tests – Prenatal tests Ability to detect IEMs prenatally has increased Biochemical methods Ø Detection of metabolites in amniotic fluid Ø Enzyme assays DNA analysis Ø Detection of genetic mutations

Prenatal tests: Choice of sample can be dictated by which disorder is to be tested for. ØAmniocentesis ØBest carried out at 15 -16 weeks ØUsed for analysis of specific metabolites by gas chromatography with mass spectroscopy, tandem mass spectroscopy, etc ØUsed for detection genetic defects using DNA technology ØIntended for diagnosis of some amino acid disorders, lysosomal storage disorders etc.

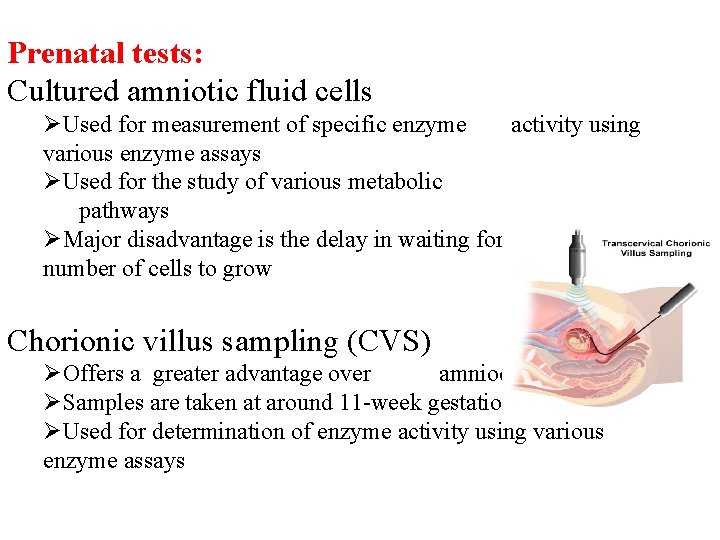
Prenatal tests: Cultured amniotic fluid cells ØUsed for measurement of specific enzyme activity using various enzyme assays ØUsed for the study of various metabolic pathways ØMajor disadvantage is the delay in waiting for sufficient number of cells to grow Chorionic villus sampling (CVS) ØOffers a greater advantage over amniocentesis ØSamples are taken at around 11 -week gestation ØUsed for determination of enzyme activity using various enzyme assays

Prenatal tests: Foetal blood and Foetal tissue ØFoetal blood is rarely used ØSample taken late in pregnancy ØUsed when there has been a failure in amniotic fluid analysis ØLiver biopsies are used when enzyme deficiencies are not expressed in CVS ØVery risky ØUsed for diagnosis of conditions where enzyme deficiency is expressed in the liver Testing of Pre-implantation embryos

Postnatal Tests • The investigation of IEM should begin with simple urine and blood analysis. • Screening tests allow you to detect the presence of a particular class of conditions and includes: ØSerum electrolytes (looking for evidence of acidosis), glucose & ammonia levels ØBlood and urine amino acids for disorders of amino acid metabolism ØUrine organic acids for disorders of organic acid metabolism, Acylcarnitine profile for disorders of fatty acid ØBlood lactate and pyruvate for disorders of carbohydrate metabolism and mitochondrial disorders


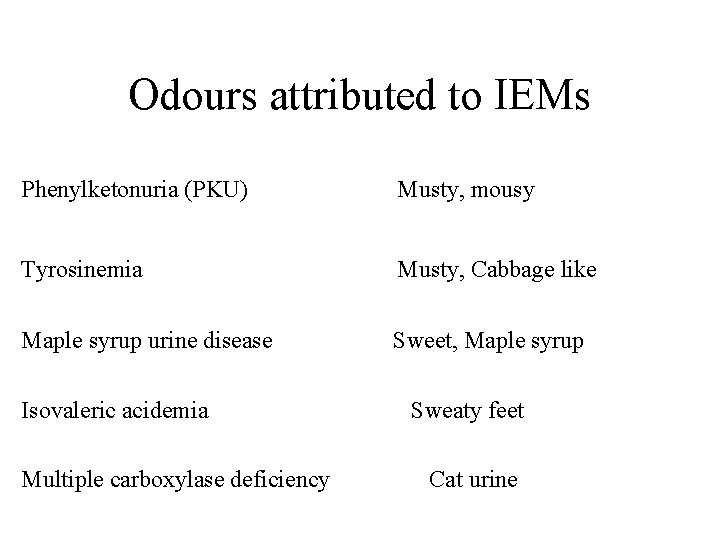
Odours attributed to IEMs Phenylketonuria (PKU) Musty, mousy Tyrosinemia Musty, Cabbage like Maple syrup urine disease Sweet, Maple syrup Isovaleric acidemia Multiple carboxylase deficiency Sweaty feet Cat urine

Examples of screening tests Tandem mass spectroscopy • Used for measurement of amino acids and acylcarnitines in blood • Used for detection of disorders of amino-acid, organic acid and fatty-acid metabolism. • Potential of simultaneous multi-disease screening • Blood taken from newborn babies are absorbed by filter paper (can also be used in the Guthrie test). • A punched sample from the dried blood spot is extracted with solvent containing appropriate isotopes • The extracted metabolites are identified and quantified with electrospray ionisation

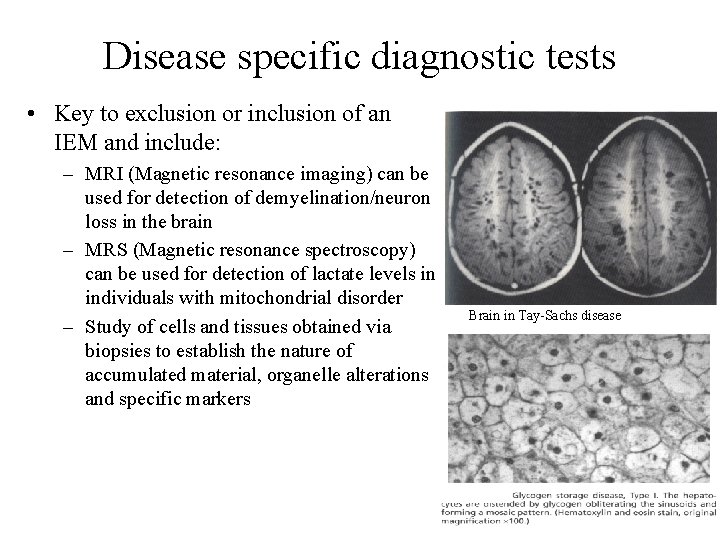
Disease specific diagnostic tests • Key to exclusion or inclusion of an IEM and include: – MRI (Magnetic resonance imaging) can be used for detection of demyelination/neuron loss in the brain – MRS (Magnetic resonance spectroscopy) can be used for detection of lactate levels in individuals with mitochondrial disorder – Study of cells and tissues obtained via biopsies to establish the nature of accumulated material, organelle alterations and specific markers Brain in Tay-Sachs disease

COLLECTIVELLY 1. 2. 3. 4. 5. Gas chromatography mass spectrometry (GCMS) of urine. High performance liquid chromatography (HPLC): for quantitative analysis of amino acids in blood and urine; required for diagnosis of organic acidemias and aminoacidopathies. Lactate/pyruvate ratio: in cases with elevated lactate. Enzyme assay: This is required for definitive diagnosis, but not available for most IEM’s. Available enzyme assays include: biotinidase assay- in cases with suspected biotinidase deficiency. Mutation analysis when available.

Precautions to be observed while collecting samples 1. 2. 3. 4. Should be collected before specific treatment is started or feeds are stopped, as may be falsely normal if the child is off feeds. Samples for blood ammonia and lactate should be transported in ice and immediately tested. Lactate sample should be arterial and should be collected after 2 hrs fasting in heparinized syringe. Ammonia sample is to be collected approximately after 2 hours of fasting in EDTA vacutainer. Detailed history including drug details should be provided to the lab.

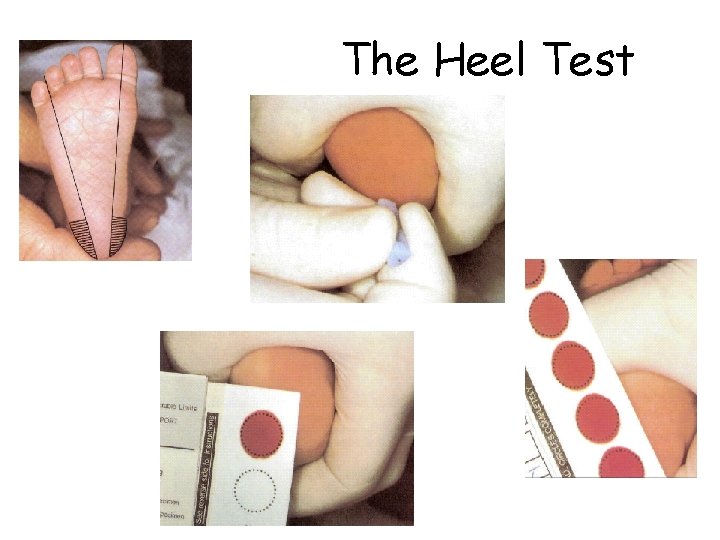
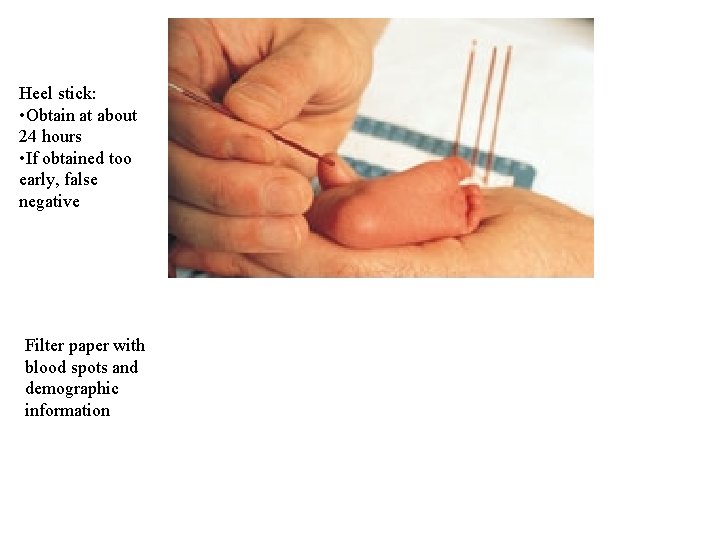
The Heel Test

“Guthrie cards” Heel stick: • Obtain at about 24 hours • If obtained too early, false negative Filter paper with blood spots and demographic information

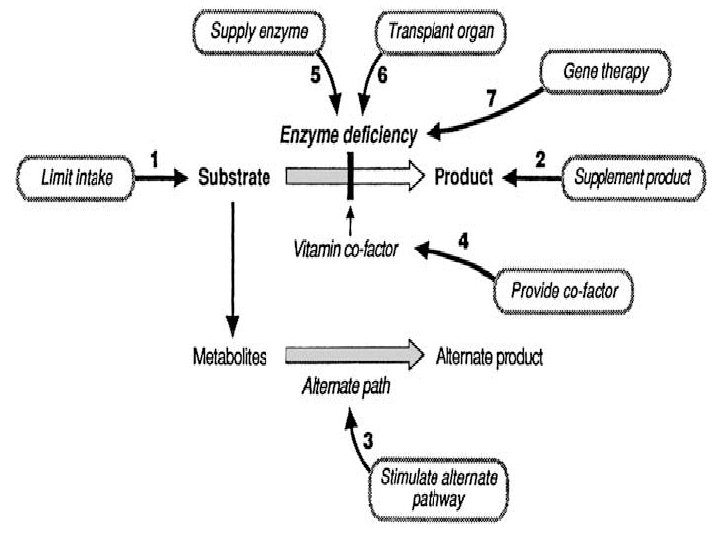
Treatments/ Management of IEMs • Treatment depends on the clinical manifestation and type of metabolites accumulated • The basic principal for treatment is reduction of the substrate that accumulates due to deficient enzyme activity • This can be mediated by an increasing number of therapeutic approaches: 1) Prevent Catabolism 2) Limit the intake of the offending substance 3) Increase excretion of toxic metabolites 4) Enzyme-replacement therapy 5) Increase the residual enzyme activity 6) Reduce substrate synthesis 7) Replacement of the end products 8) Transplantation and gene therapy


Treatments/ Management of IEMs 1) Prevent Catabolism Ø Controlling the administration of calories: used to prevent endogenous protein breakdown and induction of anabolism 2) Limit the intake of offending substance Ø Restriction of certain dietary components. Ø E. g. restriction of intake of galactose and fructose to prevent galactosaemia and fructose intolerance Ø E. g. Neonates with PKU should be given protein substitute that is phenyalanine-free. 3) Increase the excretion of toxic metabolites Ø Rapid removal of toxic metabolites can be achieved by exchange transfusion, peritoneal dialysis, haemodialysis, forced diuresis etc. Ø E. g. Haemodialysis is considered mandatory for hyperammonaemia

Treatments/ Management of IEMs 4) Enzyme replacement therapy Ø Replacement of the deficient enzyme Ø E. g. Human alpha glucosidase enzyme is used for treatment of pompe’s disease 5) Increase the residual enzyme activity (if possible) Ø Usually accomplished by administration of pharmacological doses of vitamin cofactor for the defective enzyme 6) Reduce substrate synthesis Ø Inhibiting the synthesis of a substrate that can not be converted to the end products Ø E. g used for treating lysosomal storage disorders in order to reduce the rate of glycosphingolipid breakdown.

Treatments/ Management of IEMs 7) Replacement of end products Ø Replacement of a product due to an enzyme defect Ø E. g. in patients with glycogen storage disease, hypoglycaemia is prevented with frequent feeds during the day and nasogastric feeding during night in infants and young children. 8) Transplantation and gene therapy Ø Bone marrow transplantation (BMT) has been used as effective therapy for selected IEMs Ø Mainly Lysosomal storage diseases and peroxisomal disorders are treated by BMT. Ø The main rationale is based on provision of correcting enzymes by donor cells within and outside the blood compartment. Ø In most gene therapy procedures a "normal" gene is inserted into the genome to replace an "abnormal, " disease-causing gene

What to do for the Dying Infant Suspected of Having an IEM • • • PM EXAMINATION Autopsy-performed within 4 hours of death Tissue and body fluid samples – Blood, URINE, CSF (ventricular tap), aqueous humour, skin biopsy, muscle and liver--frozen in liquid nitrogen • Filter paper discs from newborn screen