Lecture No 3 Lipids Metabolism Dr Obeid Shanab

Lecture No. 3 Lipids Metabolism Dr. Obeid Shanab
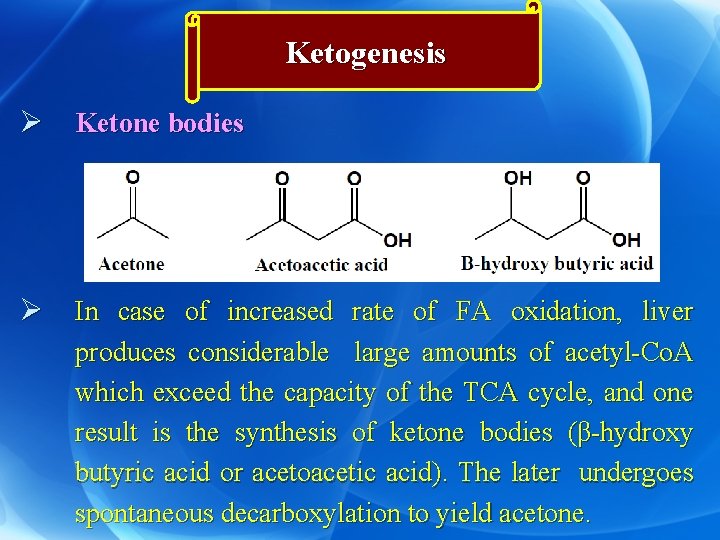
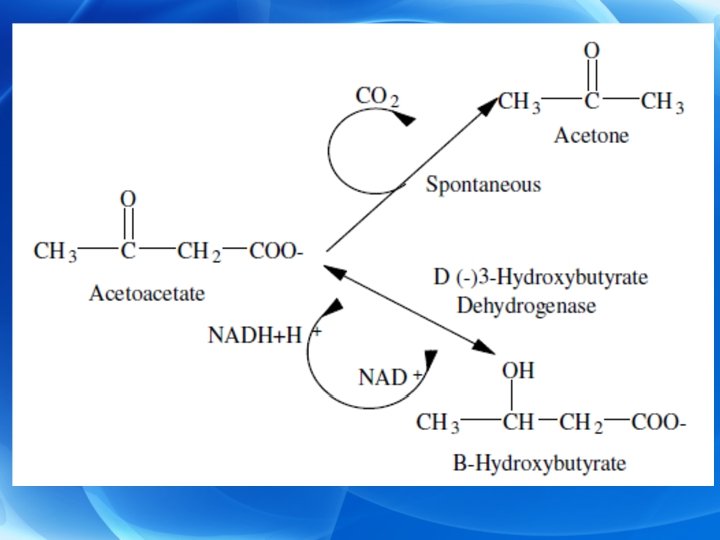
Ketogenesis Ø Ketone bodies Ø In case of increased rate of FA oxidation, liver produces considerable large amounts of acetyl-Co. A which exceed the capacity of the TCA cycle, and one result is the synthesis of ketone bodies (β-hydroxy butyric acid or acetoacetic acid). The later undergoes spontaneous decarboxylation to yield acetone.
Ø Site of ketogenesis: • Mainly in the mitochondria of liver (it contains the enzymes of ketogenesis). • Extrahepatic tissues utilize ketone bodies as fuel substrates. • The net flow of ketone bodies from the liver to the extrahepatic tissues results from an active enzymatic mechanism in the liver for the production of ketone bodies.
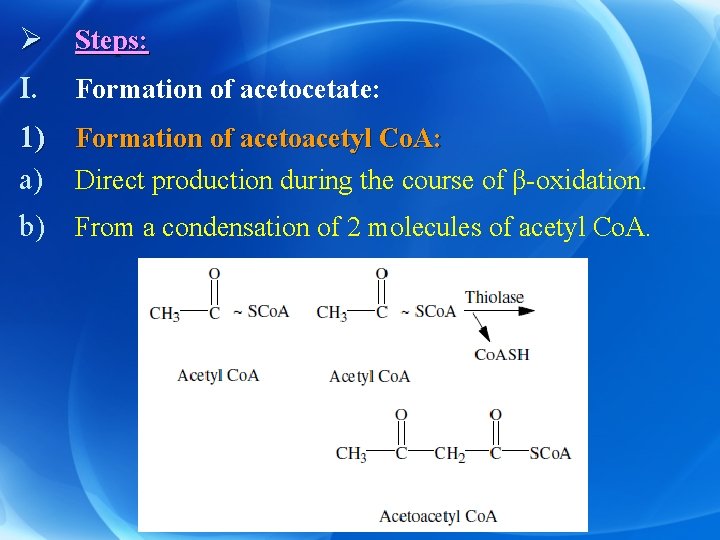
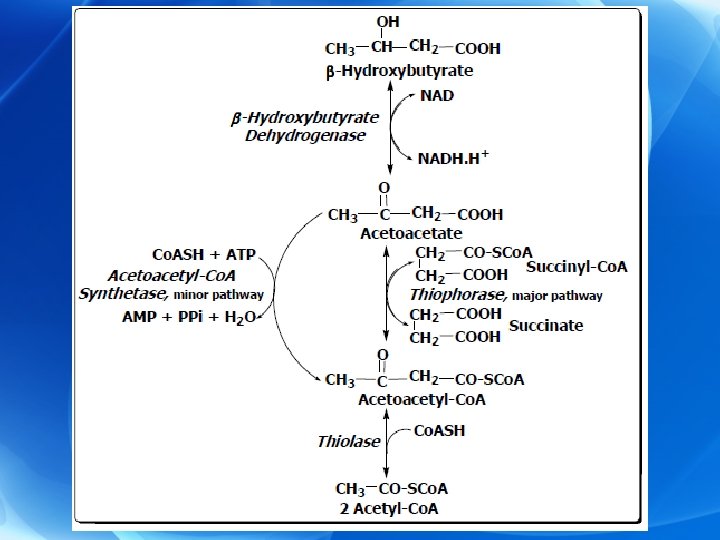
Ø Steps: I. Formation of acetocetate: 1) Formation of acetoacetyl Co. A: a) Direct production during the course of β-oxidation. b) From a condensation of 2 molecules of acetyl Co. A.

2) Conversion of acetoacetyl Co. A to acetoacetate: • Through two pathways a) Simple deacylation of acetoacetyl Co. A to acetoacetic acid (minor pathway). b) Condensation of acetoacetyl Co. A with acetyl Co. A: (major pattway).
II. Formation of β-hydroxybutyrate: • From acetoacetate by β-hydroxybutyrate dehydrogenase and NADH+H+ as coenzyme. The enzyme is present in mitochondria of many tissues, e, g. , liver. III. Formation of acetone: • By spontaneous decarboxylation of acetoacetate and is lost through lungs and in urine. • In untreated diabetes, patient’s breath and urine have acetone odor that indicate ketonemia. • Ketone bodies after being formed diffuse from the liver into the blood (ketonaemia).
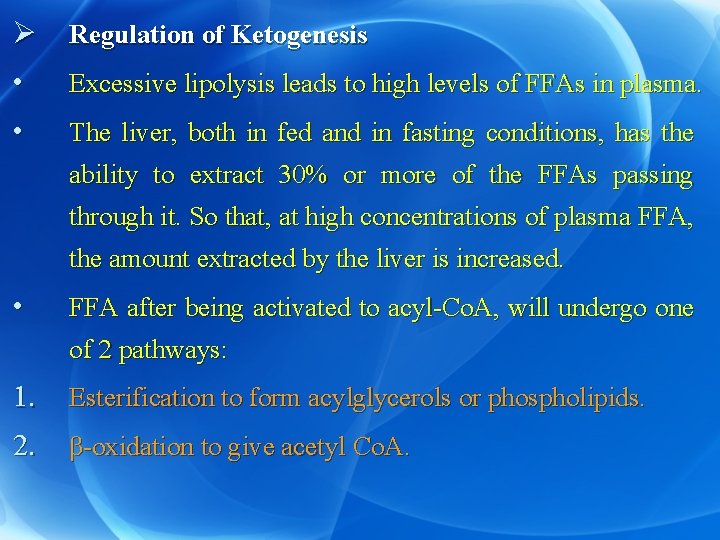
Ø Regulation of Ketogenesis • Excessive lipolysis leads to high levels of FFAs in plasma. • The liver, both in fed and in fasting conditions, has the ability to extract 30% or more of the FFAs passing through it. So that, at high concentrations of plasma FFA, the amount extracted by the liver is increased. • FFA after being activated to acyl-Co. A, will undergo one of 2 pathways: 1. Esterification to form acylglycerols or phospholipids. 2. β-oxidation to give acetyl Co. A.

Ø After meal: (ketogenesis is inhibited) a) Insulin hormone inhibits lipolysis with subsequent decrease in the level of plasma FFA. • This results in decreased uptake of FFA by the liver and consequently decreased ketogenesis. b) In the liver: esterification is stimulated while βoxidation is inhibited.

Ø This is due to: • Increased formation of glycerol-3 -phosphate which is essential for esterification. • Stimulation of insulin hormone which stimulates acyl transferase enzyme causing stimulation of esterification. • Decreased activity of carnitine acyl transferase in the mitochondria. This decreases the entry of acyl groups into the mitochondria producing decreased β-oxidation. • Inhibition of β-oxidation leads to decrease formation of acetyl Co. A which is essential for ketogenesis.

Ø During starvation: (ketogenesis is stimulated) • Stimulation of glucagon secretion which in turn stimulates lipolysis in adipose tissue with release of FFA in blood. • As the level of FFA is raised, more FFA are taken up by the liver and more FFA are converted to acetyl Co. A. • Acetyl Co. A either undergoes further oxidation in Krebs cycle or forms ketone bodies.
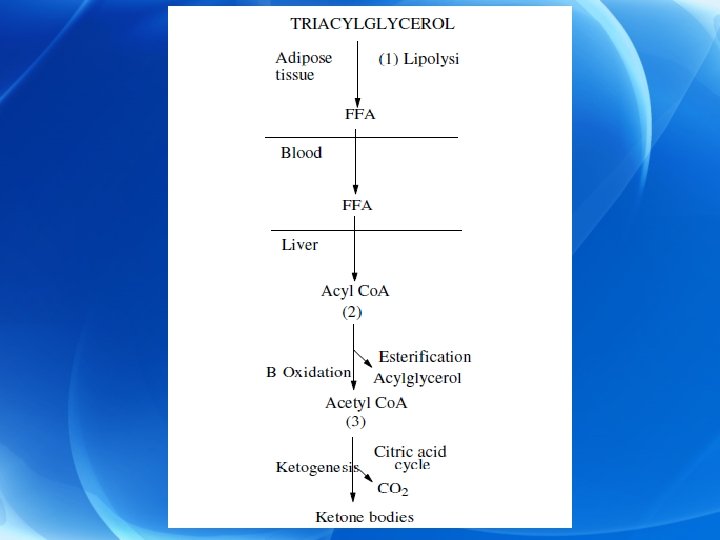

Ø This depends upon the amount of energy present in the cell: a) If there is excess ATP molecules, acetyl Co. A forms ketone bodies (one molecule of plamitate gives 33 mol of ATP on conversion to acetoacetate). b) If there is decreased ATP mol in the cell, acetyl Co. A will undergo further oxidation in Krebs cycle (one molecule of plamitate gives 129 mol of ATP on oxidation via Krebs cycle).
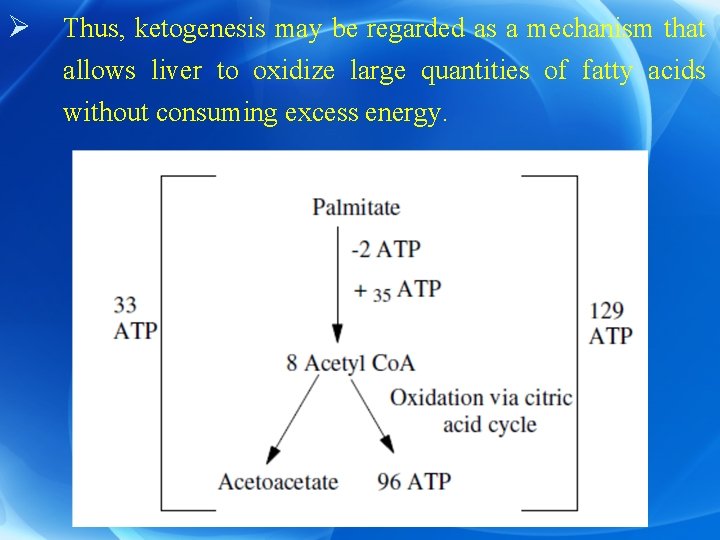
Ø Thus, ketogenesis may be regarded as a mechanism that allows liver to oxidize large quantities of fatty acids without consuming excess energy.

Ketolysis Ø It means utilization of ketone bodies. Ø Site: mainly in liver (however, liver is unable to metabolize the ketone bodies because it does not contain the enzymes responsible for the activation of ketone bodies; thiophorase and acetoacetate thiokinase enzymes). Ø Therefore, ketone bodies diffuse from liver mitochondria into the blood to be transported for extrahepatic tissues as important sources of energy as in skeletal muscles, heart. Ø In prolonged starvation brain obtain 75% of its energy from oxidation of acetoacetate.

Ø Steps of ketolysis 1) Activation of acetoacetate to acetoacetyl Co. A. a) Major pathway: • Thiophorase transfers Co. A from succinyl Co. A to acetoacetate giving rise to acetoacetyl Co. A. b) Minor pathway: • Activation of acetoacetate with ATP in the presence of Co. A catalyzed by acetoacetyl-Co. A synthetase.

2) Activation of β-hydroxybutyrate: Ø β-hydroxybutyrate may be activated directly in extrahepatic tissues by a synthetase (limited pathway). Ø However, conversion to acetoacetate followed by activation to acetoacetyl Co. A is the more important route leading to its further metabolism. Ø The acetoacetyl Co. A formed by these reactions is split to acetyl Co. A by thiolase and oxidized in citric acid cycle. 3) Acetone is lost it is in urine and breath.
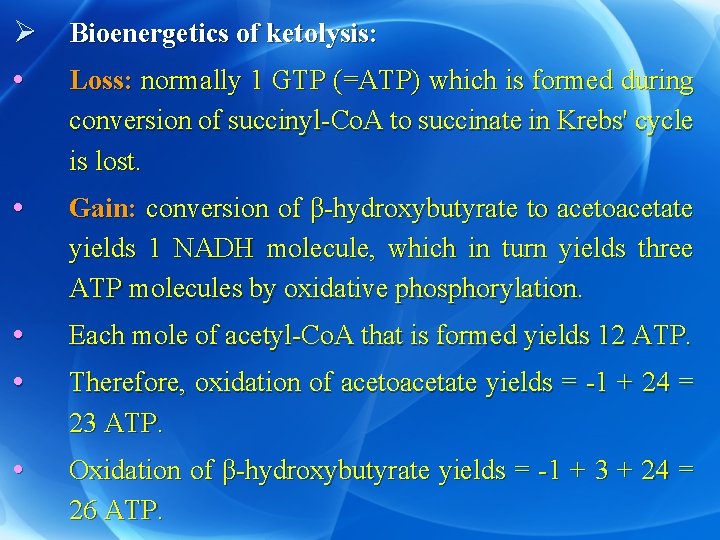
Ø Bioenergetics of ketolysis: • Loss: normally 1 GTP (=ATP) which is formed during conversion of succinyl-Co. A to succinate in Krebs' cycle is lost. • Gain: conversion of β-hydroxybutyrate to acetoacetate yields 1 NADH molecule, which in turn yields three ATP molecules by oxidative phosphorylation. • • Each mole of acetyl-Co. A that is formed yields 12 ATP. • Oxidation of β-hydroxybutyrate yields = -1 + 3 + 24 = 26 ATP. Therefore, oxidation of acetoacetate yields = -1 + 24 = 23 ATP.

Ø Ketosis • It is the increased levels of ketone bodies in blood above the normal level (0. 3 - 2 mg/d. L) leading to ketonemia associated with increased urine excretion of ketone bodies (ketonuria). • The urinary excretion of ketone bodies is usually less than one mg/24 hours. Ø Ketoacidosis • It is a type of metabolic acidosis which is due to ketosis. • Since ketone bodies are moderately strong acids when their levels are increased they are neutralized by blood buffers mainly bicarbonate (HCO 3 -).
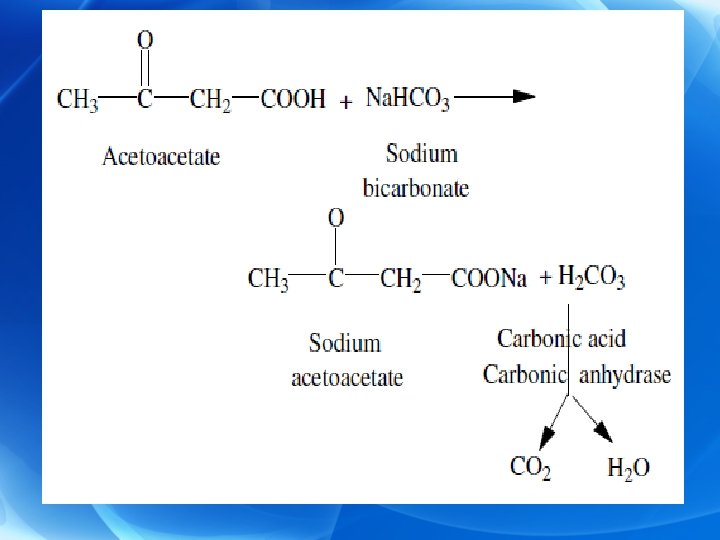

Ø This depletes HCO 3 - and as a result blood p. H is decreased. Ø Decreased blood p. H may cause transfer of K+ ions from intracellular fluid to blood causing hyperkalemia. Ø In severe cases of ketosis coma may be developed. Ø In advanced cases as in uncontrolled diabetes, the condition may be fatal.

Ø Causes of Ketosis: It is mainly due to high rate of FAs oxidation so, liver produces excess amounts of ketone bodies; These conditions are: I. • Non pathological: • High fat, low- Carbohydrate diet: Fat should be 30 %, the carbohydrate should be 58% and the protein should be 12% of the total calorie intake per day. • • Severe exercise in the postabsorptive state. Starvation: due to depletion of carbohydrate, excessive liplysis and β-oxidation of fatty acids. Prolonged ether anesthesia.

II. Pathological conditions: • Diabetes mellitus. • Renal glucosurea and phlorhizin poisning. Ø Mechanism : • Lipolysis causes high blood FFA level that go through β -oxidation then ketogenesis. • Therefore, factors controlling lipolysis can in turn control the extent of ketosis.
Lipogenesis Next lecture? ?

- Slides: 26