LECTURE Disperse systems The methods of preparing of



















- Slides: 59

LECTURE Disperse systems. The methods of preparing of colloidal solutions. Their properties. Physical-chemical properties of biopolymer solutions. ass. prof. Iryna R. Bekus

Plan 1. The main concepts and determination 2. Classification of the dispersed systems 3. Preparation methods of the dispersed systems 4. Purification methods of the dispersed systems

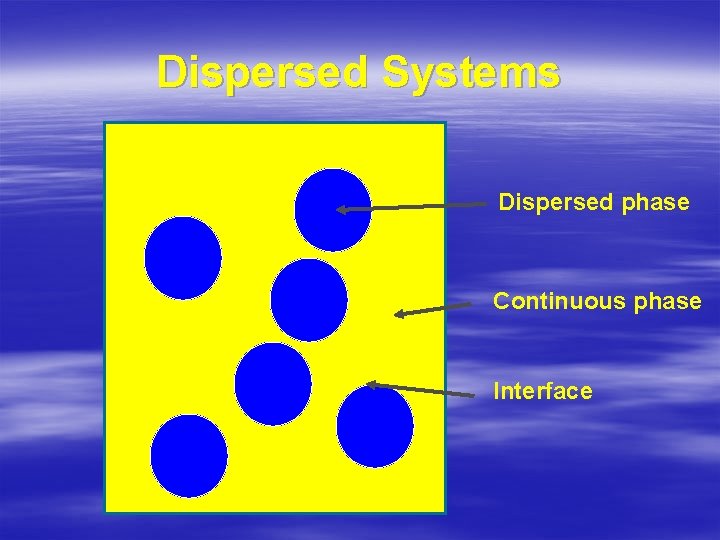
Dispersed Systems § A kinetically stable mixture of one phase in another largely immiscible phase. Usually at least one length scale is in the colloidal range.

Dispersed Systems Dispersed phase Continuous phase Interface

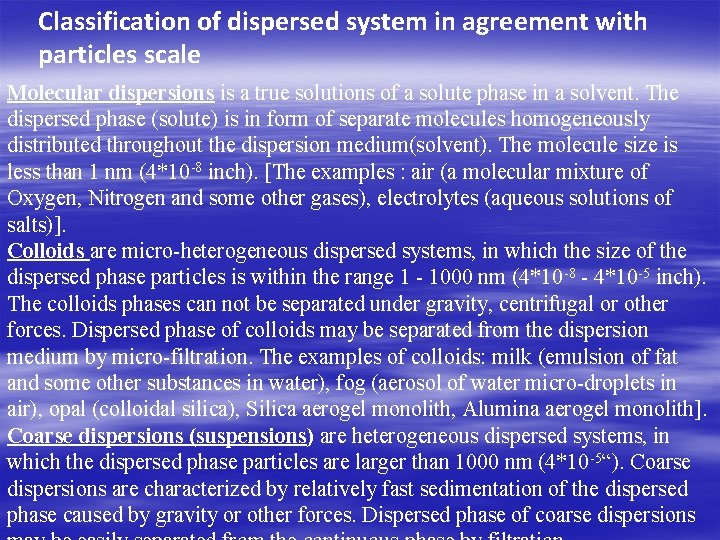
Classification of dispersed system in agreement with particles scale Molecular dispersions is a true solutions of a solute phase in a solvent. The dispersed phase (solute) is in form of separate molecules homogeneously distributed throughout the dispersion medium(solvent). The molecule size is less than 1 nm (4*10 -8 inch). [The examples : air (a molecular mixture of Oxygen, Nitrogen and some other gases), electrolytes (aqueous solutions of salts)]. Colloids are micro-heterogeneous dispersed systems, in which the size of the dispersed phase particles is within the range 1 - 1000 nm (4*10 -8 - 4*10 -5 inch). The colloids phases can not be separated under gravity, centrifugal or other forces. Dispersed phase of colloids may be separated from the dispersion medium by micro-filtration. The examples of colloids: milk (emulsion of fat and some other substances in water), fog (aerosol of water micro-droplets in air), opal (colloidal silica), Silica aerogel monolith, Alumina aerogel monolith]. Coarse dispersions (suspensions) are heterogeneous dispersed systems, in which the dispersed phase particles are larger than 1000 nm (4*10 -5“). Coarse dispersions are characterized by relatively fast sedimentation of the dispersed phase caused by gravity or other forces. Dispersed phase of coarse dispersions

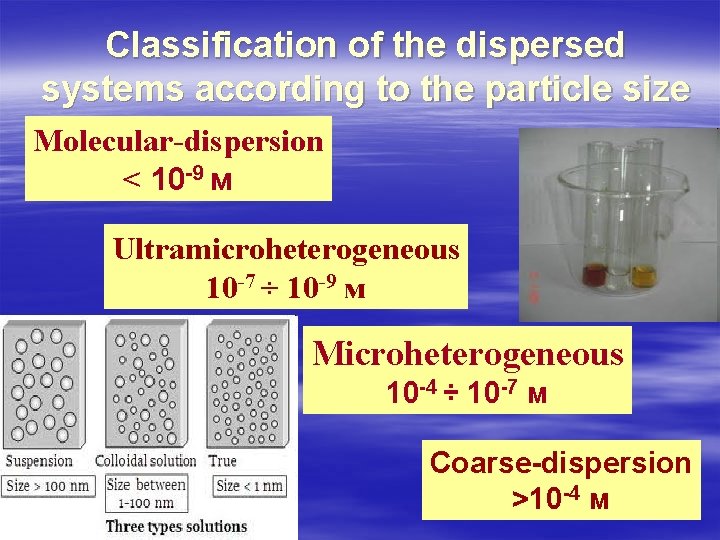
Classification of the dispersed systems according to the particle size Molecular-dispersion < 10 -9 м Ultramicroheterogeneous 10 -7 ÷ 10 -9 м Microheterogeneous 10 -4 ÷ 10 -7 м Coarse-dispersion >10 -4 м


http: //www. youtube. com/watch? v=q 96 lj. VMHYLo

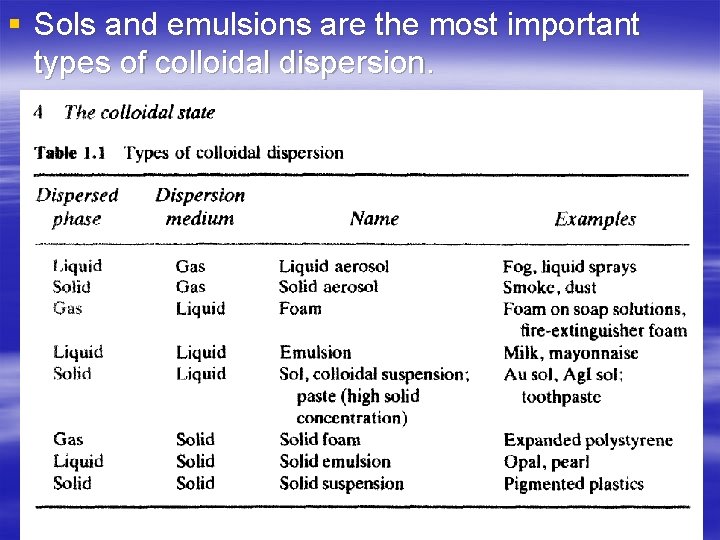
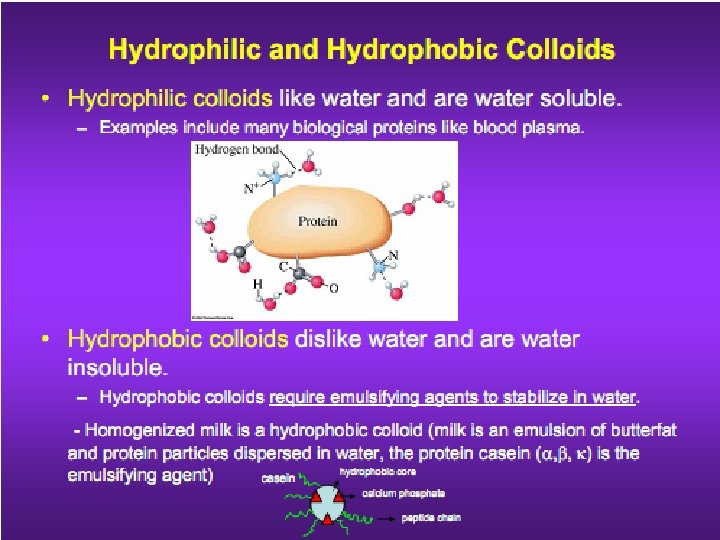
§ Sols and emulsions are the most important types of colloidal dispersion.


Fog

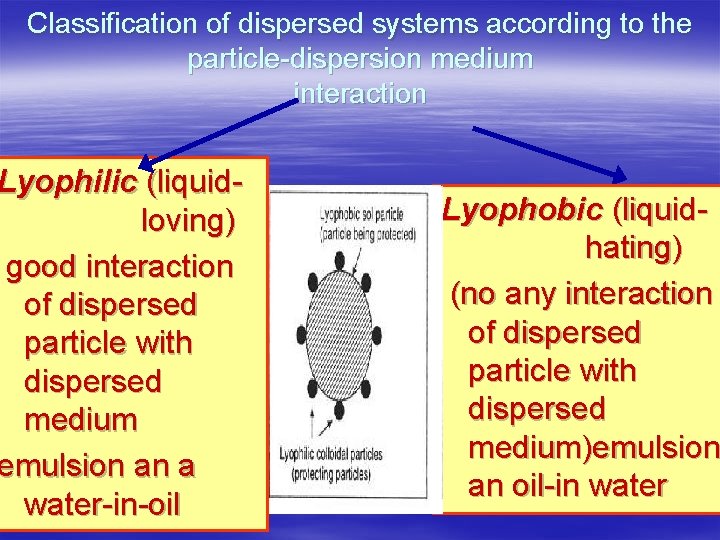
Classification of dispersed systems according to the particle-dispersion medium interaction Lyophilic (liquidloving) good interaction of dispersed particle with dispersed medium emulsion an a water-in-oil Lyophobic (liquidhating) (no any interaction of dispersed particle with dispersed medium)emulsion an oil-in water

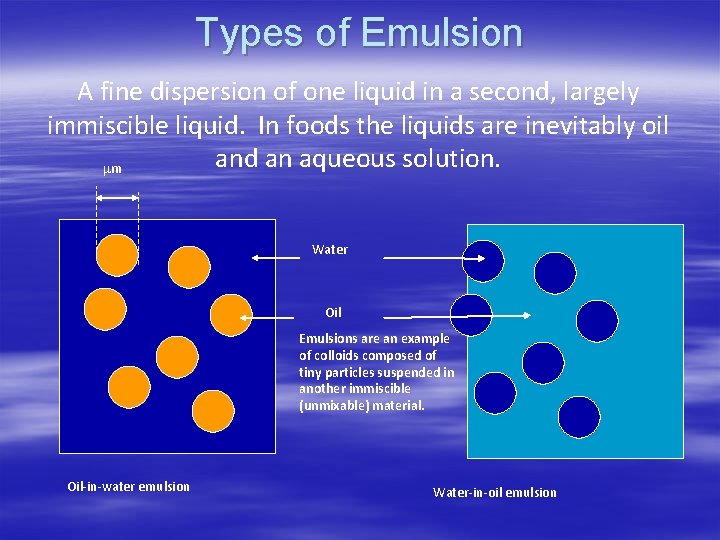
Types of Emulsion A fine dispersion of one liquid in a second, largely immiscible liquid. In foods the liquids are inevitably oil and an aqueous solution. mm Water Oil Emulsions are an example of colloids composed of tiny particles suspended in another immiscible (unmixable) material. Oil-in-water emulsion Water-in-oil emulsion


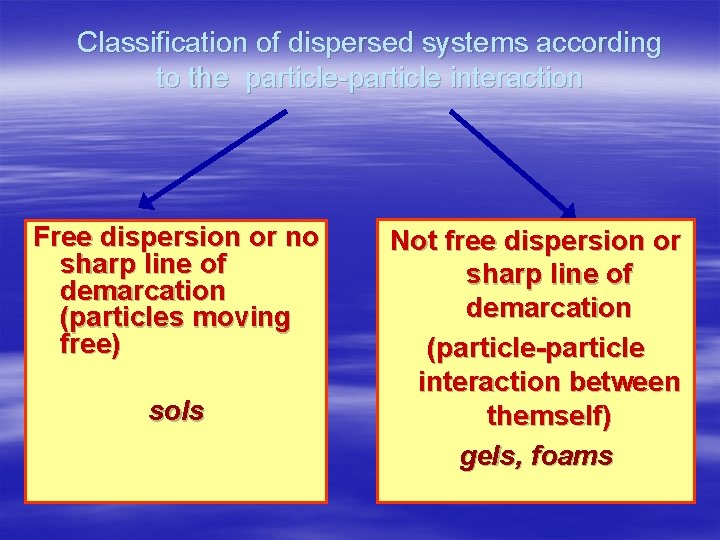
Classification of dispersed systems according to the particle-particle interaction Free dispersion or no sharp line of demarcation (particles moving free) sols Not free dispersion or sharp line of demarcation (particle-particle interaction between themself) gels, foams

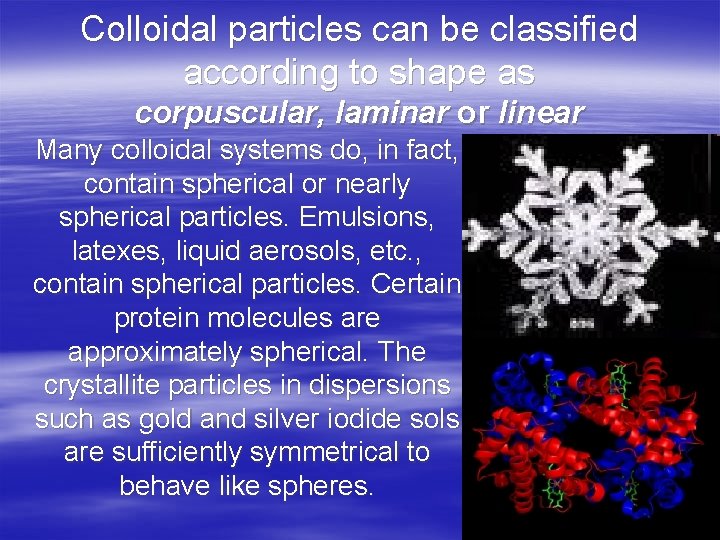
Colloidal particles can be classified according to shape as corpuscular, laminar or linear Many colloidal systems do, in fact, contain spherical or nearly spherical particles. Emulsions, latexes, liquid aerosols, etc. , contain spherical particles. Certain protein molecules are approximately spherical. The crystallite particles in dispersions such as gold and silver iodide sols are sufficiently symmetrical to behave like spheres.

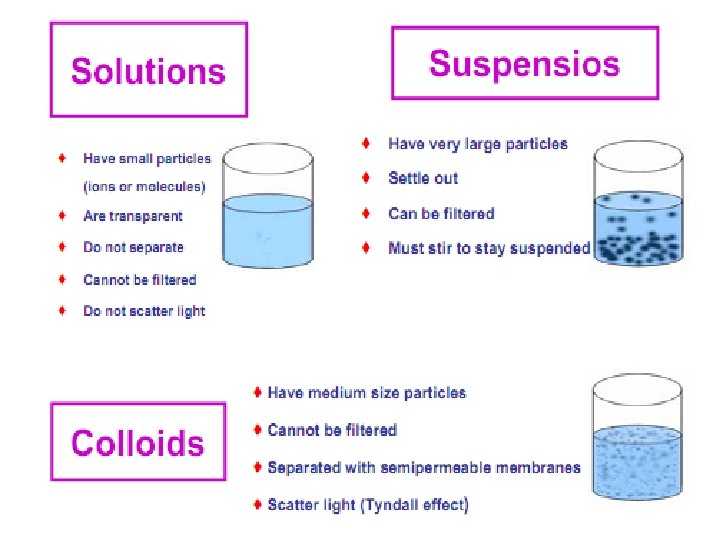
Colloidal solutions have dispersed phase particle, which size from 109 to 10 -7 m or 1 nm to 100 nm.

http: //www. youtube. com/watch? v=-j. Zyqq. N 4 uqc&feature=related

Dispersion These methods involve the breaking of the bigger particles to colloidal size.

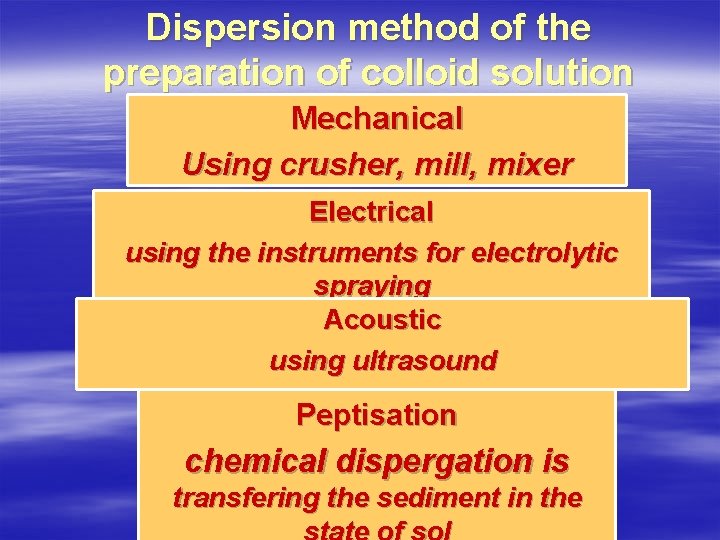
Dispersion method of the preparation of colloid solution Mechanical Using crusher, mill, mixer Electrical using the instruments for electrolytic spraying Acoustic using ultrasound Peptisation chemical dispergation is transfering the sediment in the

Peptization - is a process of passing of a precipitate into colloidal particles on adding suitable electrolyte. The electrolyte added is called peptizing agent.

Condensation methods of the preparation of the colloidal solutions. It bases on the appearing of a new phase in the homogenius phase according to the joining of molecules, atoms, ions. Physical Condensation from a pair, the substitution of a poor solvent Chemical Fe. CI 3+3 H 2 O → Fe(OH)3 +3 HCl Ag. NO 3 + KCl → Ag. Cl + KNO 3 2 H 2 S + SO 2 → 3 S + 2 H 2 O Ag 2 O + H 2 → 2 Ag + H 2 O

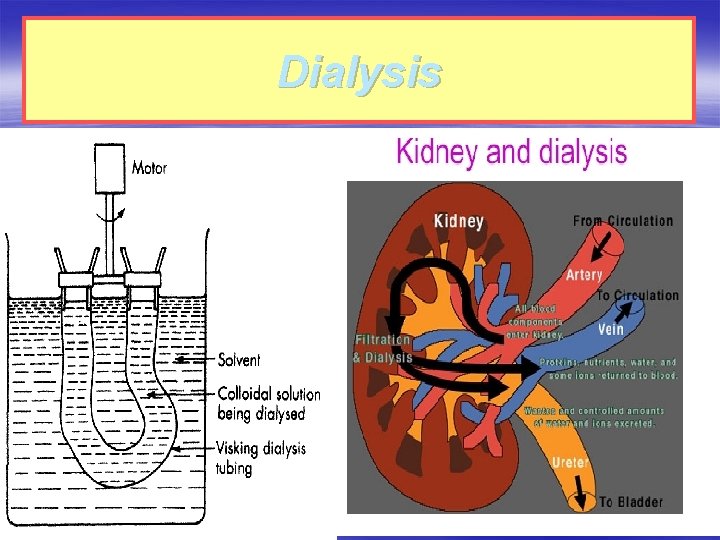
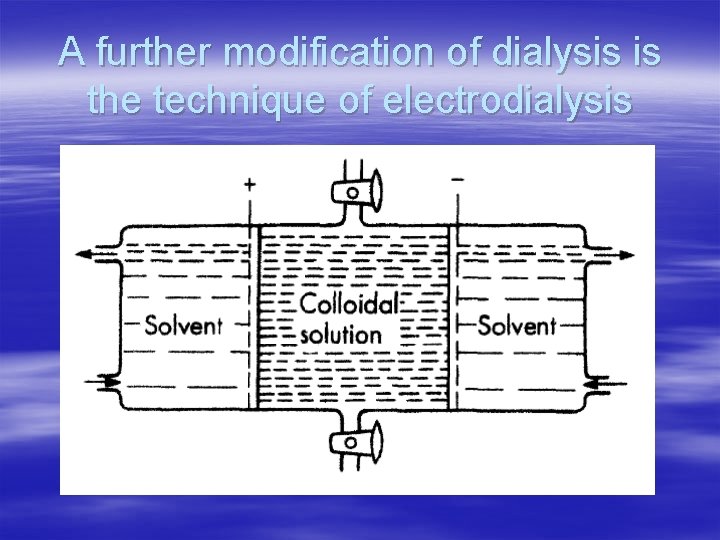
Dialysis § The process of separating the particles of colloids from those of crystalloids by diffusion of the mixture through semipermeable membrane (а parchment or an animal membrane) is known as dialysis. § The above process can be quickened if an electric field is applied around the membrane (the process is then called Electrodialysis).

Dialysis

A further modification of dialysis is the technique of electrodialysis

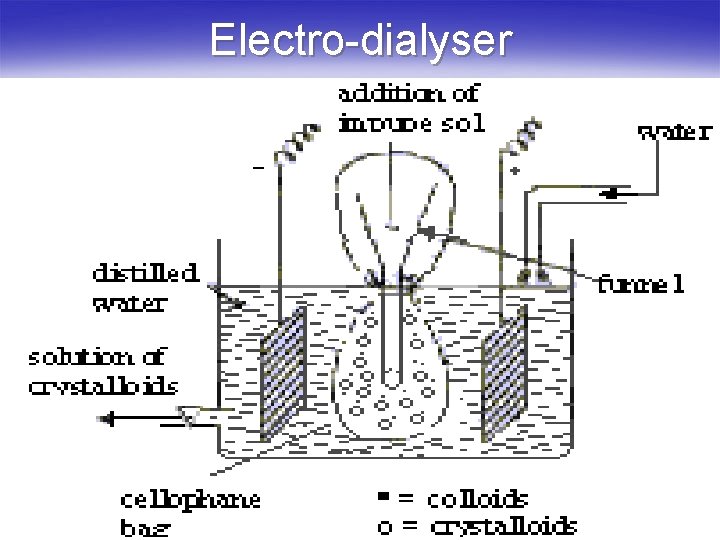
Electro-dialyser

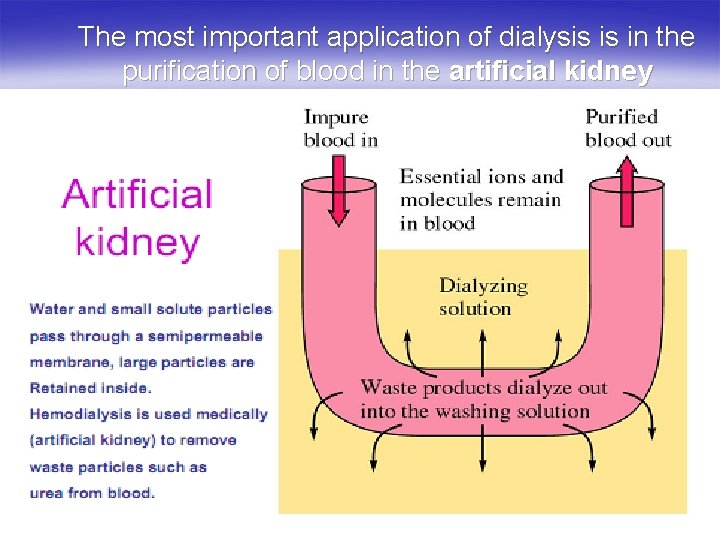
The most important application of dialysis is in the purification of blood in the artificial kidney

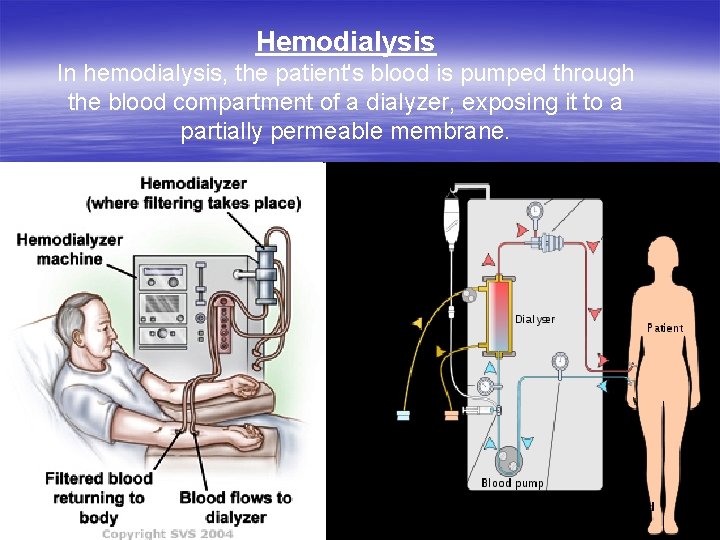
Hemodialysis In hemodialysis, the patient's blood is pumped through the blood compartment of a dialyzer, exposing it to a partially permeable membrane.

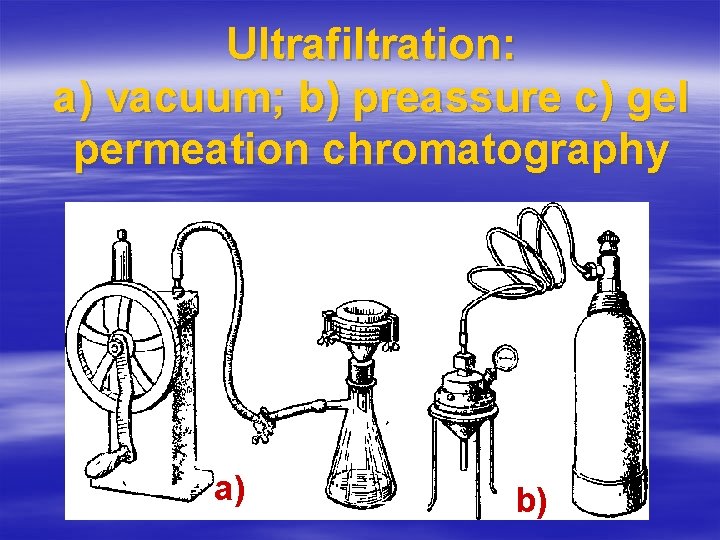
Ultrafiltration: а) vacuum; b) preassure c) gel permeation chromatography а) b)

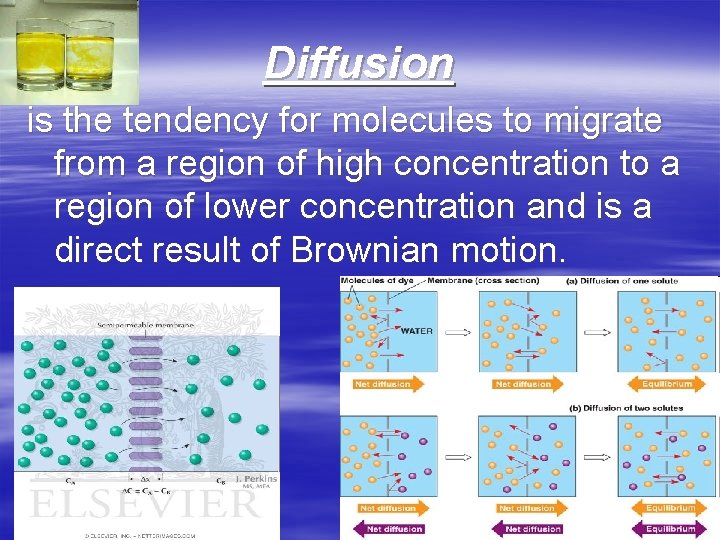
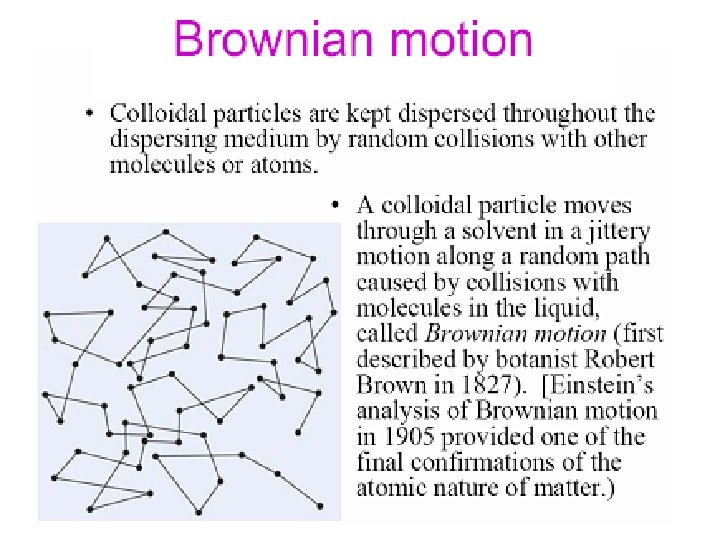
Diffusion is the tendency for molecules to migrate from a region of high concentration to a region of lower concentration and is a direct result of Brownian motion.

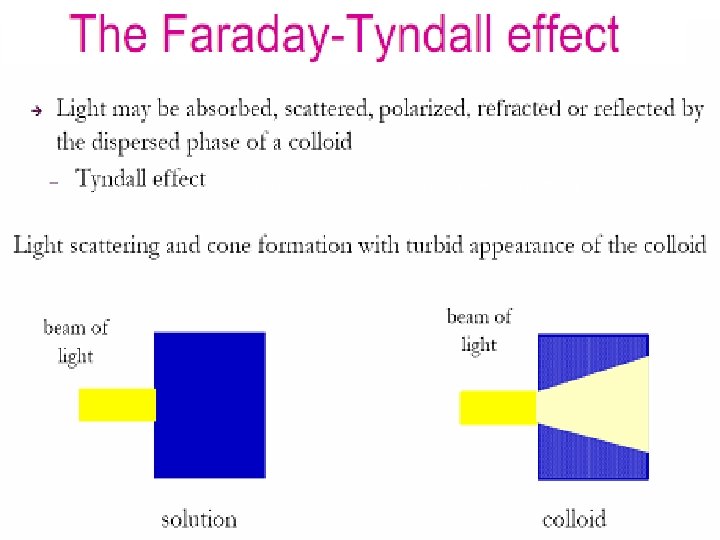
Properties 1. Physical Properties § Heterogeneous character § Stability § Filterability § Visibility 2. Colligative properties - osmotic pressure 3. Mechanical properties – Brownian movement 4. Optical properties – Tyndall affect 5. Electrical properties


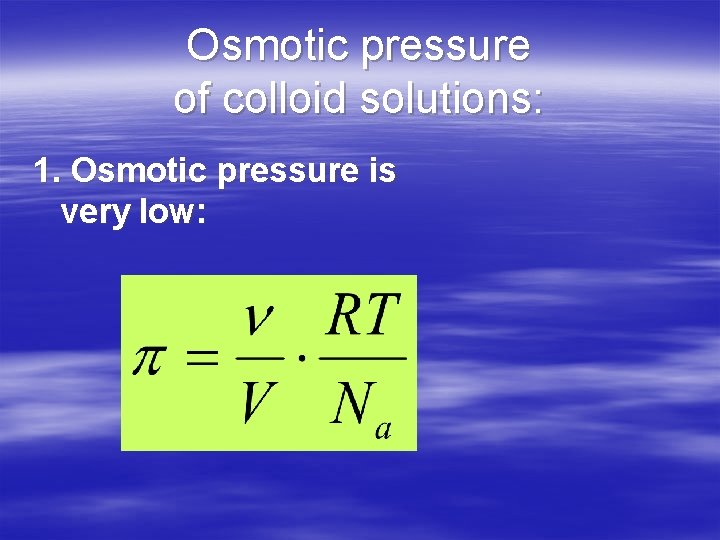
Osmotic pressure of colloid solutions: 1. Osmotic pressure is very low:

http: //www. youtube. com/watch? v=k 5 HMVIb 4 J 7 A&NR=1

Kinetic stability § А major source of kinetic stability of colloids is the existence of an electric charge on the surfaces of the particles. On the account of this charge, ions of opposite charge tend to cluster nearby, and an ionic atmosphere is formed.

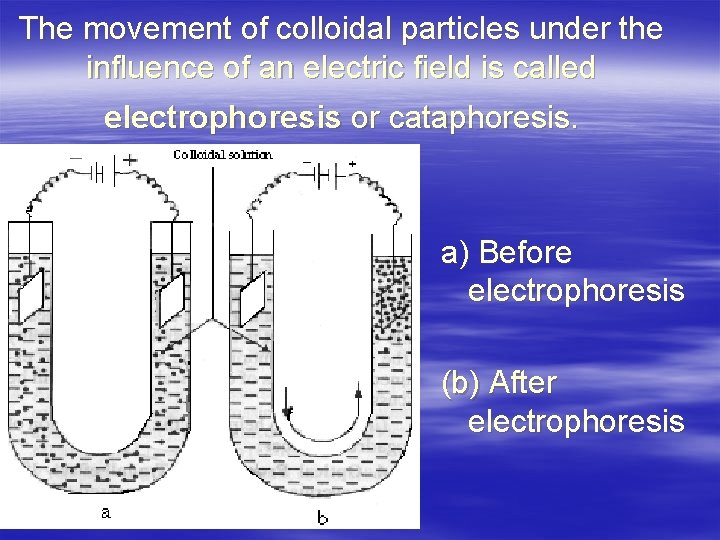
The movement of colloidal particles under the influence of an electric field is called electrophoresis or cataphoresis. а) Before electrophoresis (b) After electrophoresis

Flocculation (coagulation) Aggregation of the particles arising from the stabilizing effect of this secondary minimum is called flocculation.

Hardy-Schulze Law § Greater is the valency of oppositely charged ion of electrolyte being added, faster is the coagulation. the the

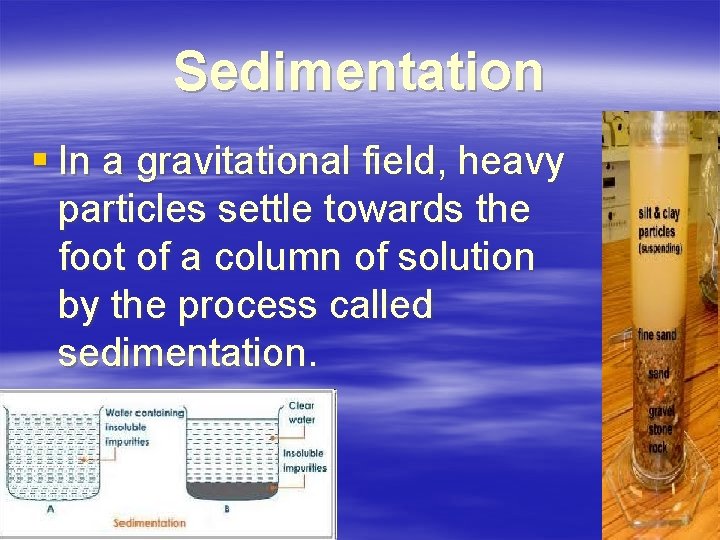
Sedimentation § In а gravitational field, heavy particles settle towards the foot of а column of solution by the process called sedimentation.

Biopolymers are polymers produced by living organisms. Since they are polymers, Biopolymers contain monomeric units that are covalently bonded to form larger structures. There are three main classes of biopolymers based on the differing monomeric units used and the structure of the biopolymer formed. Polynucleotides long polymers which are composed of 13 or more nucleotide monomers, Polypeptides short polymers of amino acids, and Polysaccharides which are often linear bonded polymeric carbohydrate structures. Cellulose is the most common organic compound and biopolymer on Earth. About 33 percent of all plant matter is cellulose. The cellulose content of cotton is 90 percent and that of wood is 50 percent.

Physical-chemical properties of biopolymers. The high-molecular compounds (HMC) are compounds – polymers, which have 10000 – 10000000 Da (Dalton – unit of atomic mass) molecular mass. А polymer is а large molecule formed by the covalent bonding of repeating smaller molecules. For example natural macromolecules: polysaccharides: glycogen, cellulose, starch; nucleic acids: RNA, DNA; proteins.

Biological role of polymers § § § § Biopolymers, have a lot functions: Catalytic effect– enzymes; As regulators – hormones; is the storage and transfer of genetic information. (DNA); Storage energy (Starch, glycogen); Protection - immunoglobulin; Structural (collagen, keratins, fibril).

CLASSIFICATION HMC § § Polymers are classified by different possible: Classification by source; Classification by structure; Classification by synthesis; Classification by molecular forces.

Classification by source § Natural (nucleic acids, polysaccharides, protein, natural rubber (polyisoprene)); § Synthetic (polyethelene, teflon, polyvinilchloride, polystyrene).

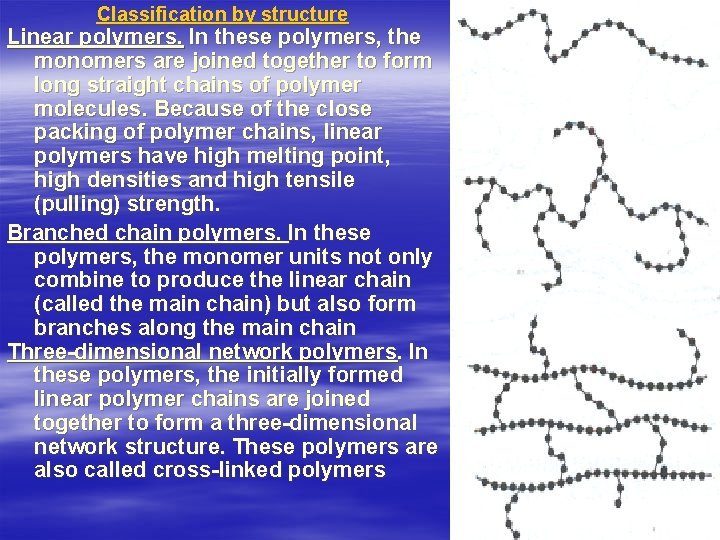
Classification by structure Linear polymers. In these polymers, the monomers are joined together to form long straight chains of polymer molecules. Because of the close packing of polymer chains, linear polymers have high melting point, high densities and high tensile (pulling) strength. Branched chain polymers. In these polymers, the monomer units not only combine to produce the linear chain (called the main chain) but also form branches along the main chain Three-dimensional network polymers. In these polymers, the initially formed linear polymer chains are joined together to form а three-dimensional network structure. These polymers are also called cross-linked polymers

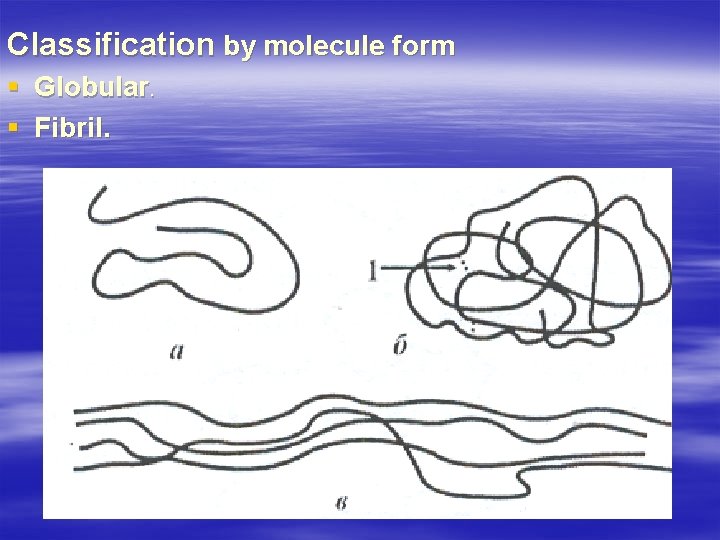
Classification by molecule form § Globular. § Fibril.

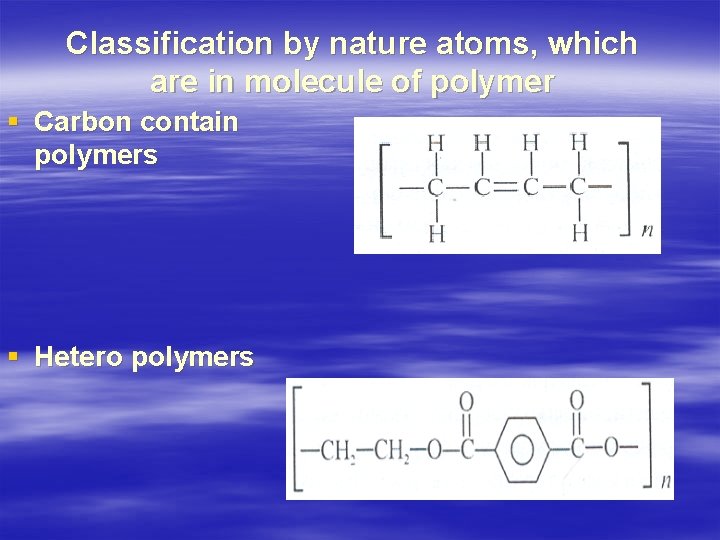
Classification by nature atoms, which are in molecule of polymer § Carbon contain polymers § Hetero polymers

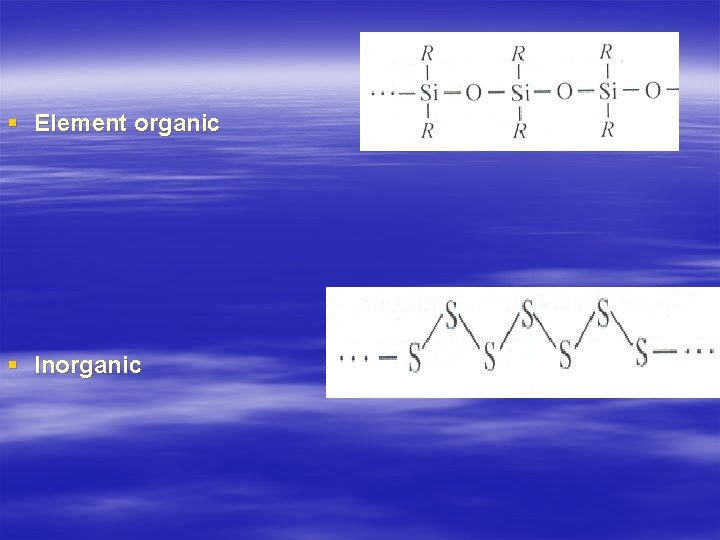
§ Element organic § Inorganic

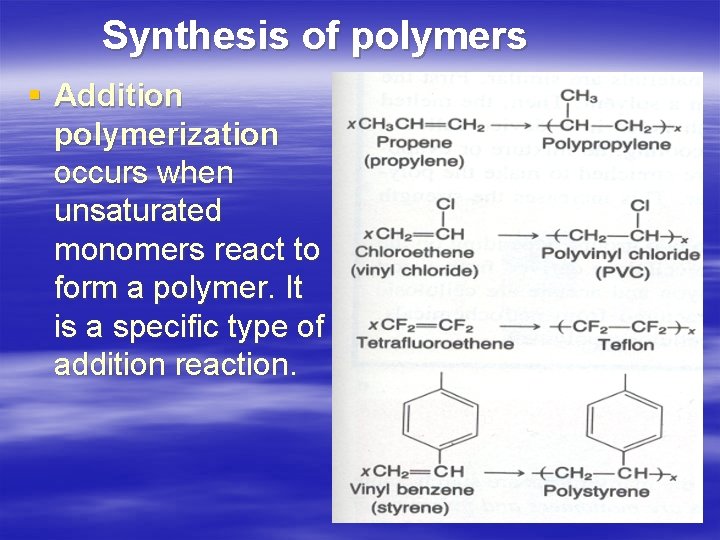
Synthesis of polymers § Addition polymerization occurs when unsaturated monomers react to form а polymer. It is а specific type of addition reaction.

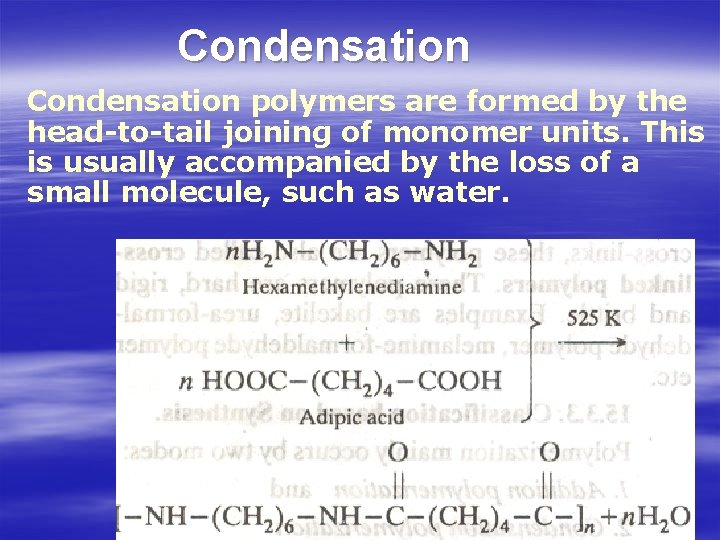
Condensation polymers are formed by the head-to-tail joining of monomer units. This is usually accompanied by the loss of а small molecule, such as water.

Properties § § § Properties HMC solution, which same as true solutions: Solutions of high-molecular compounds are stable as molecular solutions; Solutions of high-molecular compounds are convertible. If high-molecular compound was solved that the molecular solution will be farmed. And if this solution to strip to dryness, so highmolecular compound was stat, which can solve again. Between high-molecular compound and solvent has not boundary.

Properties HMC solution, which same as colloidal solutions: Size of disperse phase in solutions of highmolecular compounds are same as in colloidal solutions (10 -7 - 10 -9 m); High-molecular compounds can not permeate through semipermeable membrane; High-molecular compounds slowly are diffused in solutions. Specific properties HMC solution: For solutions of high-molecular compounds are characteristic the swelling and high viscosity

Definition of Micelles (Associated colloids). § There are some substances which at low concentrations behave as normal strong electrolytes but at higher concentrations exhibit colloidal behavior due to the formation of aggregated particles. These associated particles are called micelles or associated colloids.

Preparation of Lyophilic Sols § Since lyophilic sols are quite stable, they can be easily prepared by shaking the lyophilic substance with the dispersion medium. § Examples are: Colloidal sols of gum, starch, gelatine and egg albumin.

Preparation of Lyophobic Sols • Lyophobic sols are prepared by two methods. They are: • 1) Condensation methods - In condensation methods particles of atomic or molecular size are induced to combine to form aggregates of colloidal dimensions. To achieve this, chemical as well as physical methods are employed. • 2) Dispersion methods. - In dispersion methods, colloidal particles are obtained by breaking large particles of a substance in the presence of a dispersion medium. Since the sols formed are unstable, they are stabilized by adding stabilizing agents.

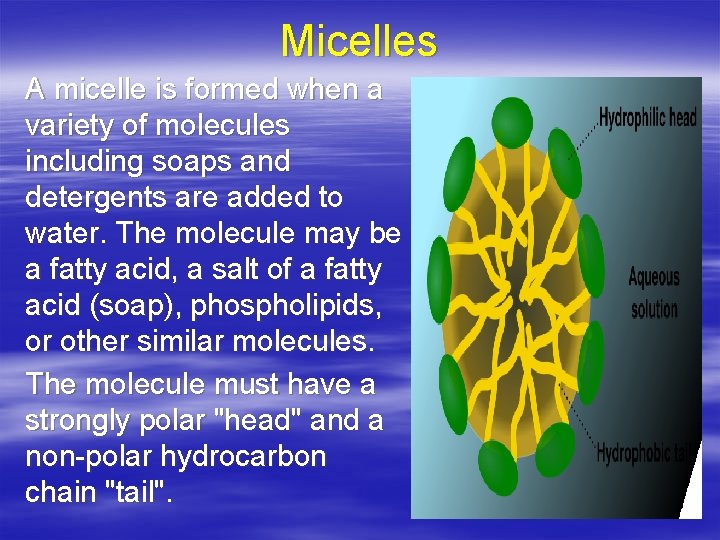
Micelles A micelle is formed when a variety of molecules including soaps and detergents are added to water. The molecule may be a fatty acid, a salt of a fatty acid (soap), phospholipids, or other similar molecules. The molecule must have a strongly polar "head" and a non-polar hydrocarbon chain "tail".

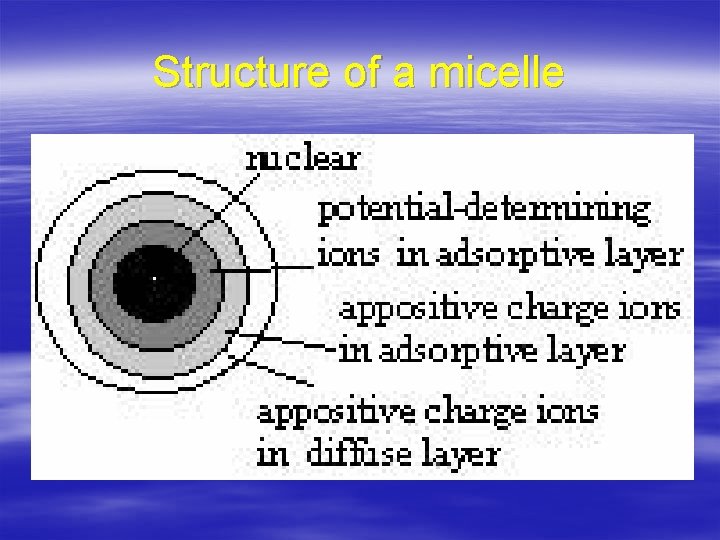
Structure of a micelle

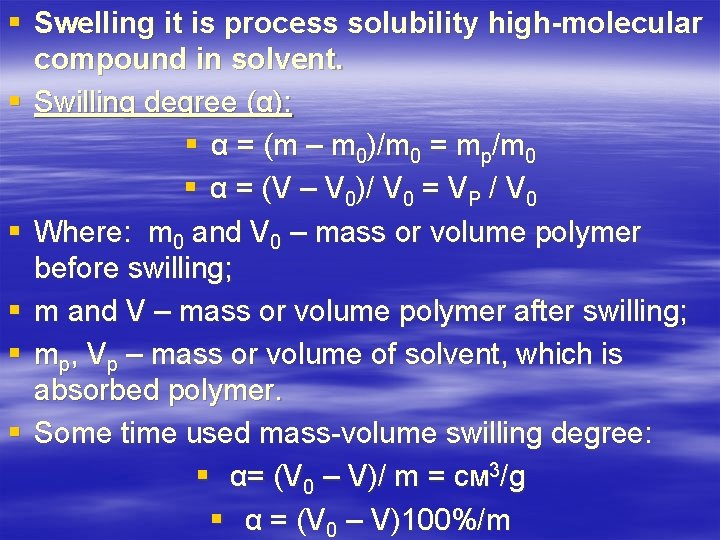
§ Swelling it is process solubility high-molecular compound in solvent. § Swilling degree (α): § α = (m – m 0)/m 0 = mp/m 0 § α = (V – V 0)/ V 0 = VP / V 0 § Where: m 0 and V 0 – mass or volume polymer before swilling; § m and V – mass or volume polymer after swilling; § mp, Vp – mass or volume of solvent, which is absorbed polymer. § Some time used mass-volume swilling degree: § α= (V 0 – V)/ m = cм 3/g § α = (V 0 – V)100%/m

Thank you for attention