Lecture about Radioactive Decay Prof Dr Shaban Harb

Lecture about Radioactive Decay Prof. Dr. Shaban Harb

Radioactive decay Radionuclides are unstable and decay by emission of particles or gamma radiation to achieve stable configuration of protons and neutrons in the nucleus. The process by which radionuclide change from unstable to stable nuclides is known as radioactive decay
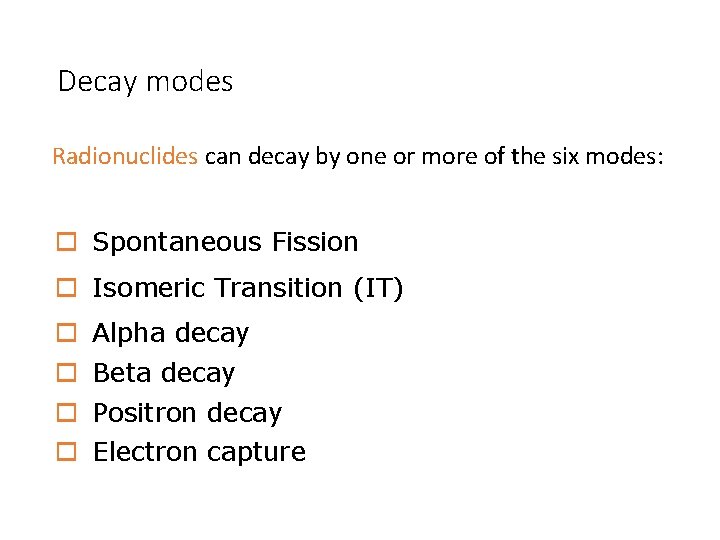
Decay modes Radionuclides can decay by one or more of the six modes: o Spontaneous Fission o Isomeric Transition (IT) o o Alpha decay Beta decay Positron decay Electron capture

Spontaneous Fission is a process in which a heavy nucleus breaks into two fragments accompanied by emission two or three neutrons
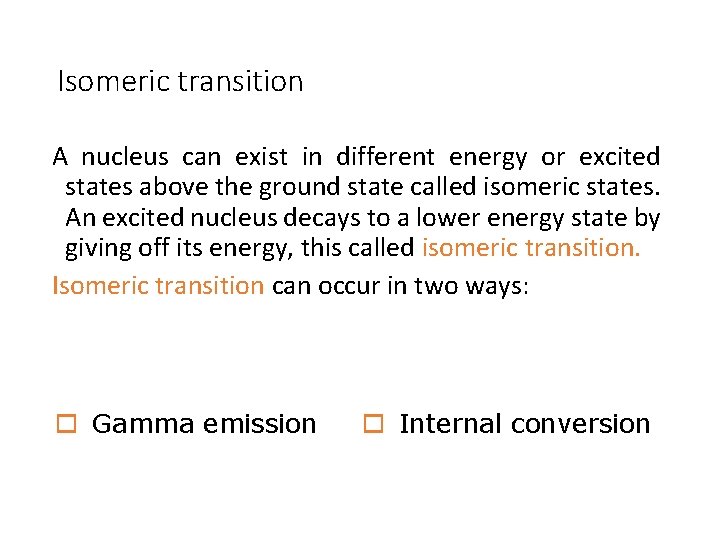
Isomeric transition A nucleus can exist in different energy or excited states above the ground state called isomeric states. An excited nucleus decays to a lower energy state by giving off its energy, this called isomeric transition. Isomeric transition can occur in two ways: o Gamma emission o Internal conversion
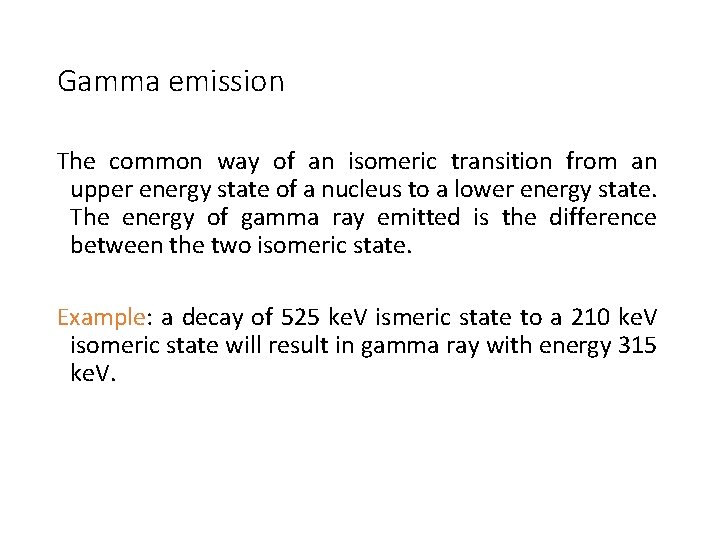
Gamma emission The common way of an isomeric transition from an upper energy state of a nucleus to a lower energy state. The energy of gamma ray emitted is the difference between the two isomeric state. Example: a decay of 525 ke. V ismeric state to a 210 ke. V isomeric state will result in gamma ray with energy 315 ke. V.

Internal Conversion The excited nucleus transfers the excitation energy to an orbital electron (K-shell electron) which then ejected from the shell provided the excitation energy is greater than the binding energy of the electron. The ejected electron is called the conversion electron.

Internal Conversion Internal conversion process leaves an atom with a vacancy in one of its shells (K shell) which filled by electron from the next higher shell such as L shell. When an L shell electron fills in a K shell vacancy, the energy difference between the two shells appears as x-ray or this energy transferred to an orbital electron which emitted with energy equal to the x-ray energy – its binding energy.

Internal Conversion These electrons are called Auger electrons and the process is termed the Auger process. Q 1. A K-shell electron is ejected by the internal conversion of a 155 ke. V gamma ray. If the binding energy of the electron is 25 ke. V. What is the kinetic energy of the electron? Q 2. can a K-shell electron be emitted as an Auger electron?
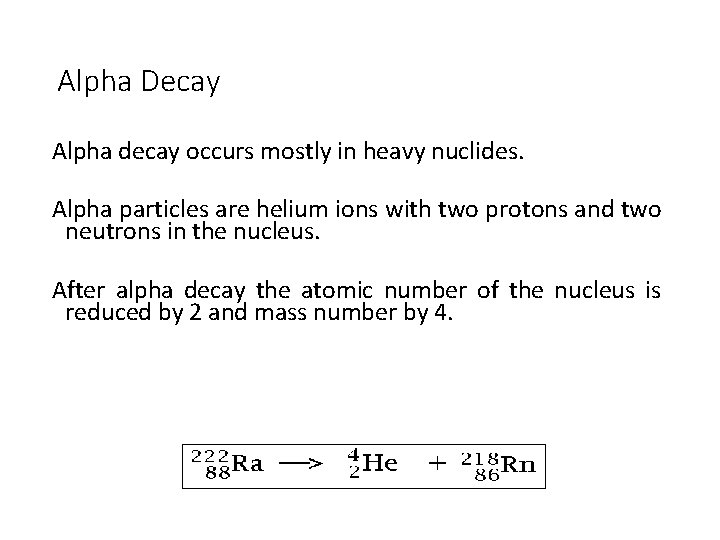
Alpha Decay Alpha decay occurs mostly in heavy nuclides. Alpha particles are helium ions with two protons and two neutrons in the nucleus. After alpha decay the atomic number of the nucleus is reduced by 2 and mass number by 4.

Alpha Decay The energy ranges of alpha particles are from 1 to 10 me. V. The range of alpha particles is very short approximately 0. 03 mm in body tissue and a few centimeters in air.
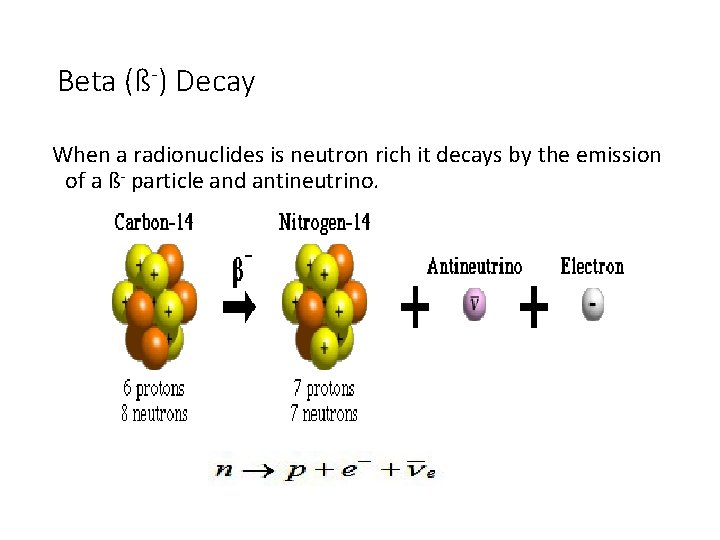
Beta (ß-) Decay When a radionuclides is neutron rich it decays by the emission of a ß- particle and antineutrino.

Beta (ß-) Decay In ß- decay the atomic number of the product increase by 1 and mass number unchanged. ß- particles carry all the transition energy Emax or part of it. The average energy of ßparticles is about 13 Emax.
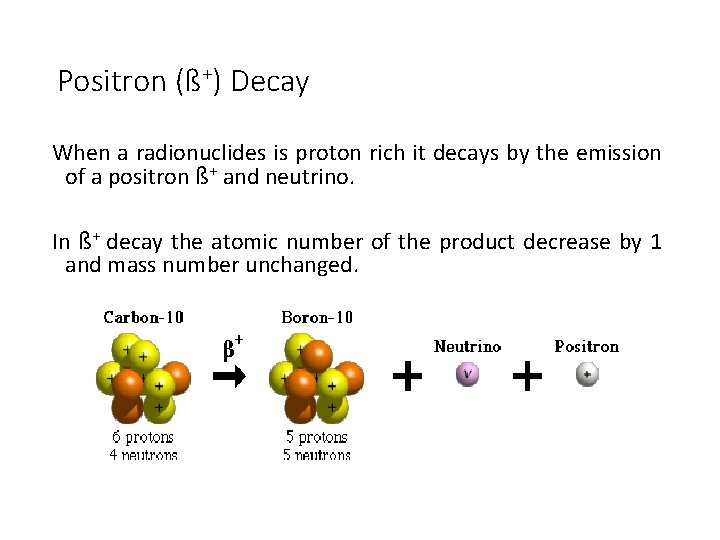
Positron (ß+) Decay When a radionuclides is proton rich it decays by the emission of a positron ß+ and neutrino. In ß+ decay the atomic number of the product decrease by 1 and mass number unchanged.

Positron (ß+) Decay ß+ emission takes place only when the transition energy is greater than 1. 02 Me. V. Annihilation radiation result from combines of ß+ with electron of the medium passing through it.
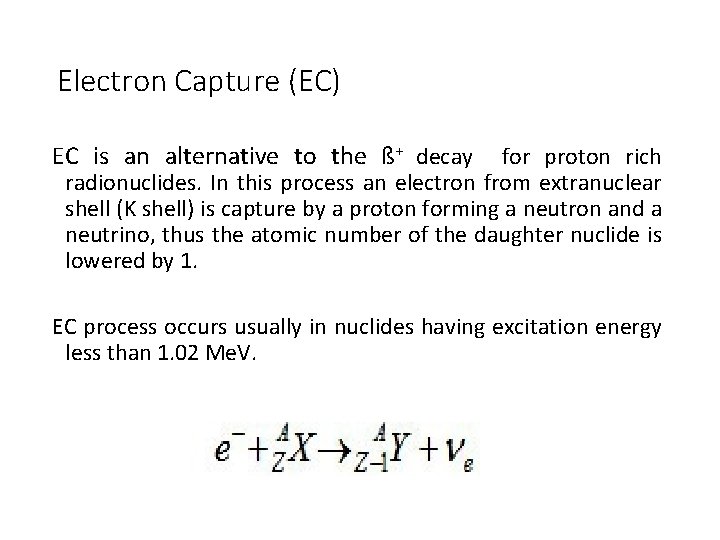
Electron Capture (EC) EC is an alternative to the ß+ decay for proton rich radionuclides. In this process an electron from extranuclear shell (K shell) is capture by a proton forming a neutron and a neutrino, thus the atomic number of the daughter nuclide is lowered by 1. EC process occurs usually in nuclides having excitation energy less than 1. 02 Me. V.

Radioactive Decay Q 1. if the energy difference is 1. 2 Me. V, could the parent radionuclide decay by ß+ or EC? If the energy difference is 0. 8 Me. V, what should be the mode of decay? Q 2. if the transition energy of ß- decay was 9 Me. V, what is the average energy of ß- particles emitted?
- Slides: 17