Lecture 9 Calculating Amounts of Reactants and Products



- Slides: 23

Lecture #9 Calculating Amounts of Reactants and Products and The Limiting Reactant Concept Chemistry 142 B James B. Callis, Instructor Autumn Quarter, 2004



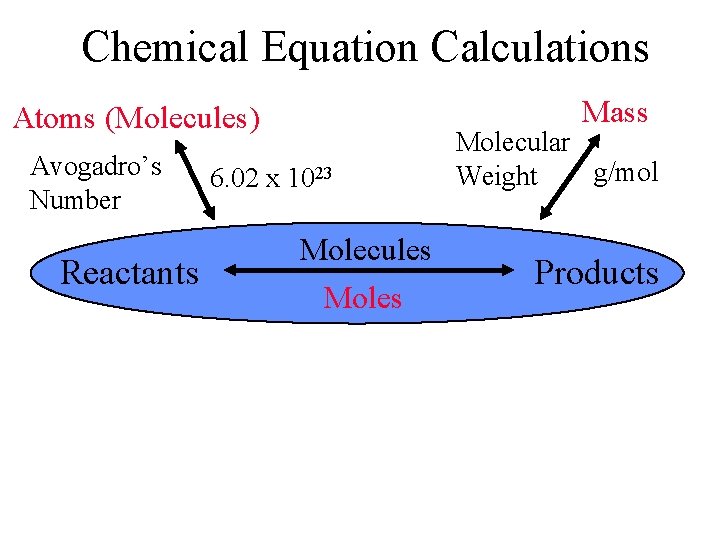
Chemical Equation Calculations Mass Atoms (Molecules) Avogadro’s Number Reactants 6. 02 x 1023 Molecules Molecular g/mol Weight Products

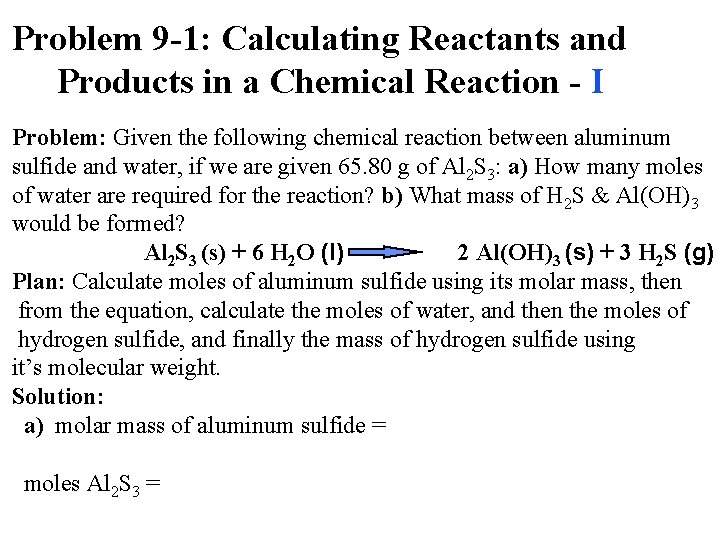
Problem 9 -1: Calculating Reactants and Products in a Chemical Reaction - I Problem: Given the following chemical reaction between aluminum sulfide and water, if we are given 65. 80 g of Al 2 S 3: a) How many moles of water are required for the reaction? b) What mass of H 2 S & Al(OH)3 would be formed? Al 2 S 3 (s) + 6 H 2 O (l) 2 Al(OH)3 (s) + 3 H 2 S (g) Plan: Calculate moles of aluminum sulfide using its molar mass, then from the equation, calculate the moles of water, and then the moles of hydrogen sulfide, and finally the mass of hydrogen sulfide using it’s molecular weight. Solution: a) molar mass of aluminum sulfide = moles Al 2 S 3 =

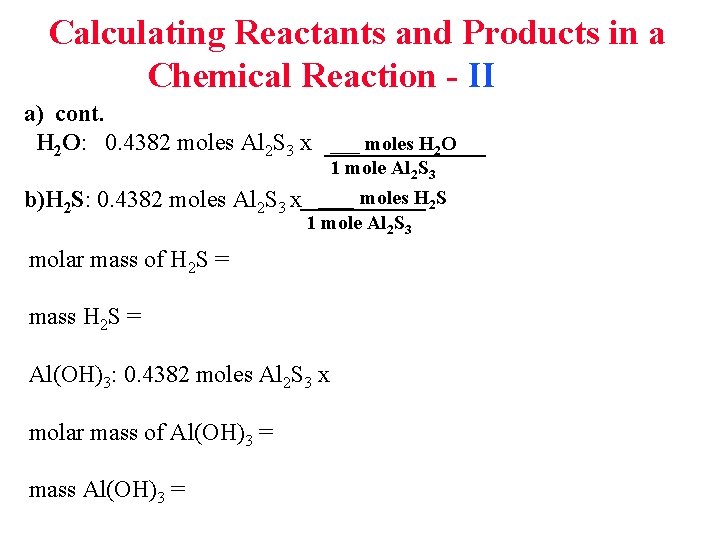
Calculating Reactants and Products in a Chemical Reaction - II a) cont. H 2 O: 0. 4382 moles Al 2 S 3 x ___ moles H 2 O b)H 2 S: 0. 4382 moles 1 mole Al 2 S 3 x ___ moles H 2 S 1 mole Al 2 S 3 molar mass of H 2 S = mass H 2 S = Al(OH)3: 0. 4382 moles Al 2 S 3 x molar mass of Al(OH)3 = mass Al(OH)3 =

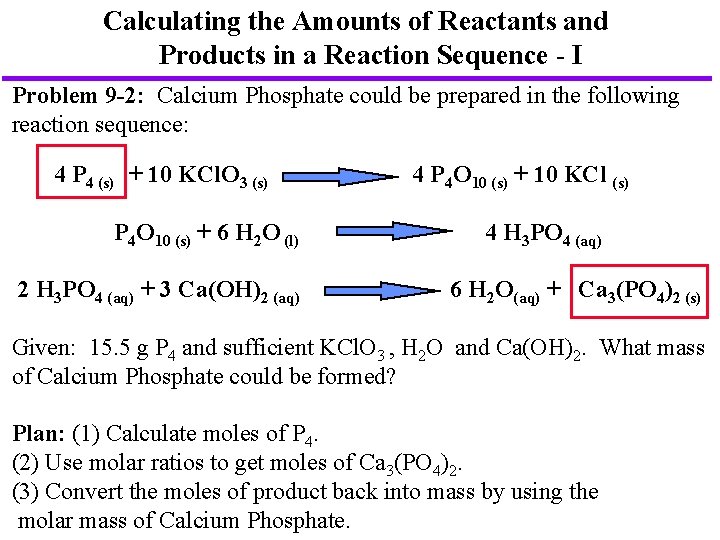
Calculating the Amounts of Reactants and Products in a Reaction Sequence - I Problem 9 -2: Calcium Phosphate could be prepared in the following reaction sequence: 4 P 4 (s) + 10 KCl. O 3 (s) P 4 O 10 (s) + 6 H 2 O (l) 2 H 3 PO 4 (aq) + 3 Ca(OH)2 (aq) 4 P 4 O 10 (s) + 10 KCl (s) 4 H 3 PO 4 (aq) 6 H 2 O(aq) + Ca 3(PO 4)2 (s) Given: 15. 5 g P 4 and sufficient KCl. O 3 , H 2 O and Ca(OH)2. What mass of Calcium Phosphate could be formed? Plan: (1) Calculate moles of P 4. (2) Use molar ratios to get moles of Ca 3(PO 4)2. (3) Convert the moles of product back into mass by using the molar mass of Calcium Phosphate.

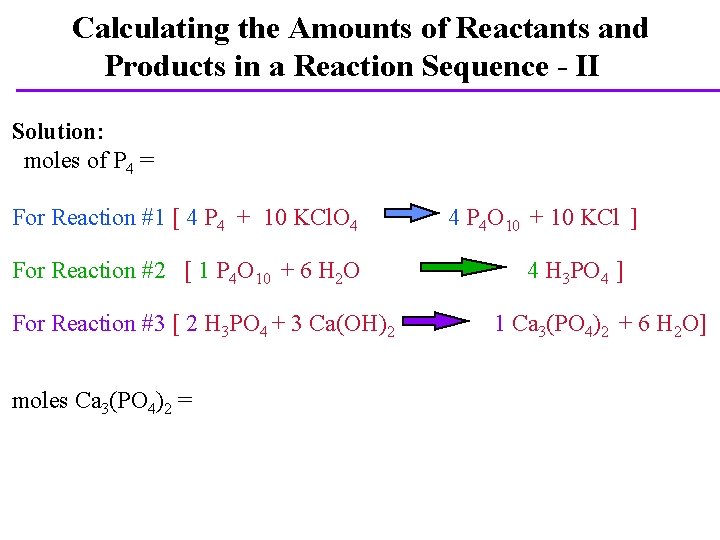
Calculating the Amounts of Reactants and Products in a Reaction Sequence - II Solution: moles of P 4 = For Reaction #1 [ 4 P 4 + 10 KCl. O 4 For Reaction #2 [ 1 P 4 O 10 + 6 H 2 O For Reaction #3 [ 2 H 3 PO 4 + 3 Ca(OH)2 moles Ca 3(PO 4)2 = 4 P 4 O 10 + 10 KCl ] 4 H 3 PO 4 ] 1 Ca 3(PO 4)2 + 6 H 2 O]

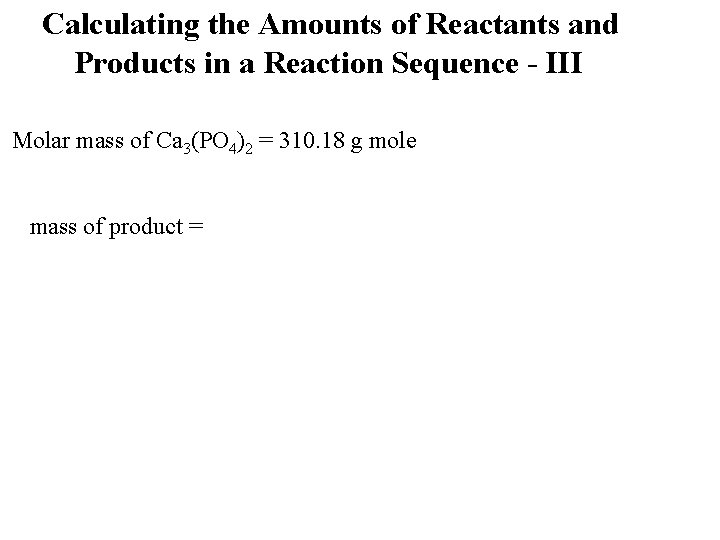
Calculating the Amounts of Reactants and Products in a Reaction Sequence - III Molar mass of Ca 3(PO 4)2 = 310. 18 g mole mass of product =

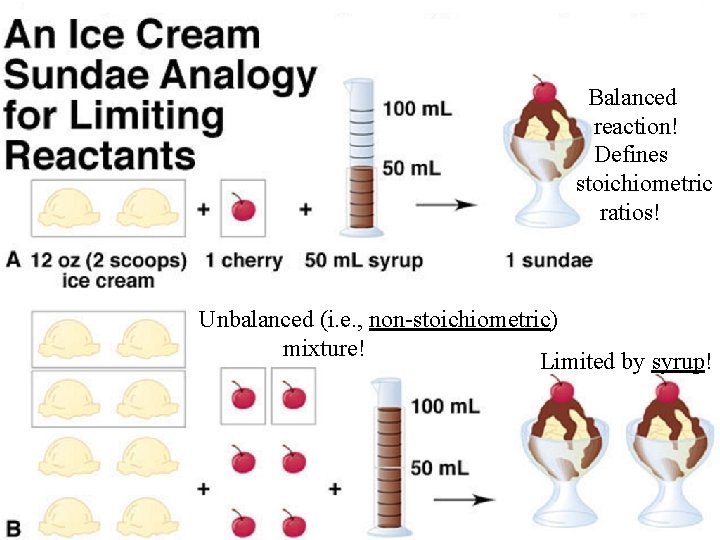
Balanced reaction! Defines stoichiometric ratios! Unbalanced (i. e. , non-stoichiometric) mixture! Limited by syrup!

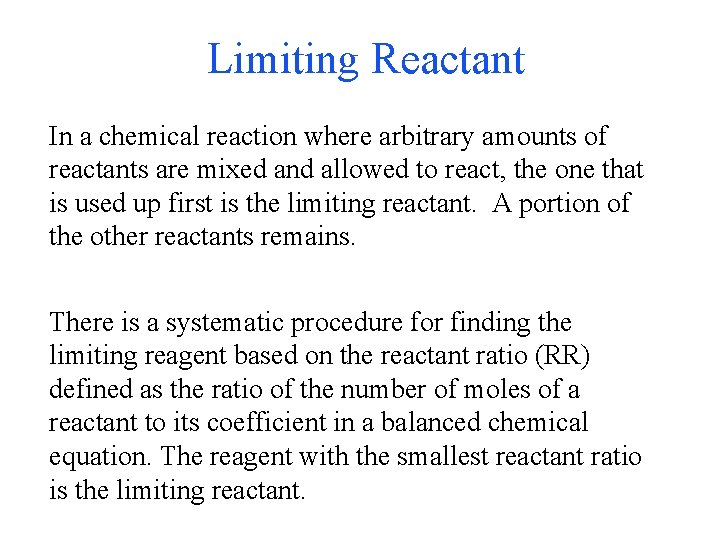
Limiting Reactant In a chemical reaction where arbitrary amounts of reactants are mixed and allowed to react, the one that is used up first is the limiting reactant. A portion of the other reactants remains. There is a systematic procedure for finding the limiting reagent based on the reactant ratio (RR) defined as the ratio of the number of moles of a reactant to its coefficient in a balanced chemical equation. The reagent with the smallest reactant ratio is the limiting reactant.

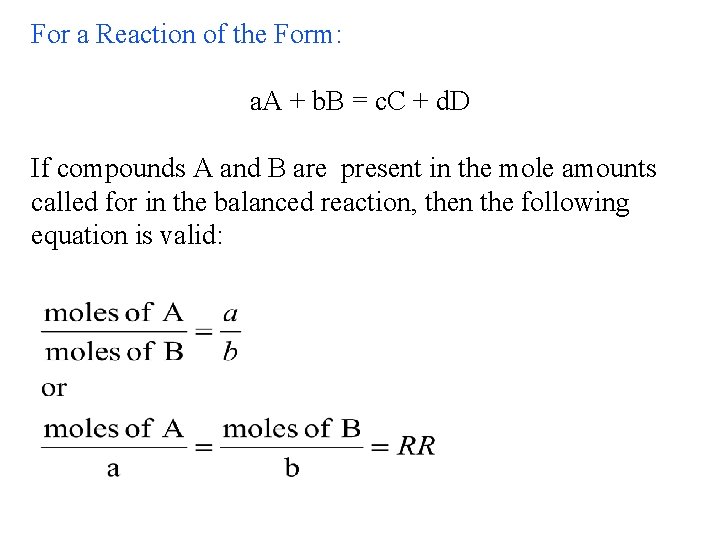
For a Reaction of the Form: a. A + b. B = c. C + d. D If compounds A and B are present in the mole amounts called for in the balanced reaction, then the following equation is valid:

If the Reactant Ratios of all reactants are equal, then there is no limiting reactant, and all reactants will be consumed. If all reactant ratios are not equal, then the reactant with the smallest reactant ratio is the limiting reactant.

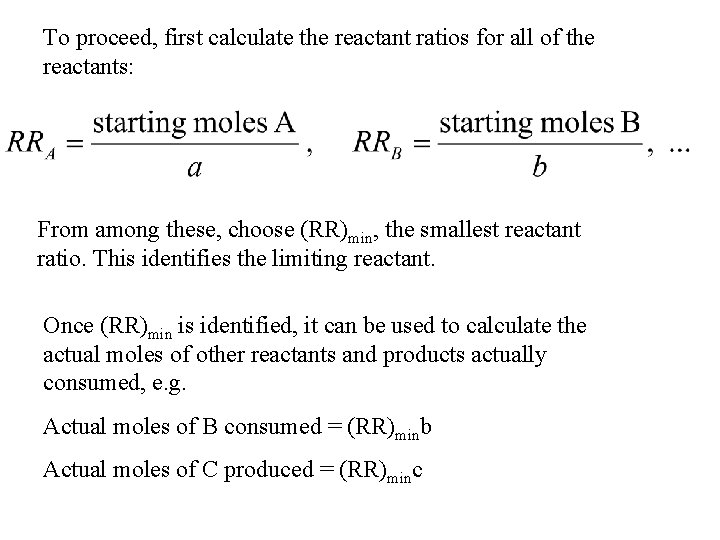
To proceed, first calculate the reactant ratios for all of the reactants: From among these, choose (RR)min, the smallest reactant ratio. This identifies the limiting reactant. Once (RR)min is identified, it can be used to calculate the actual moles of other reactants and products actually consumed, e. g. Actual moles of B consumed = (RR)minb Actual moles of C produced = (RR)minc

Problem 9 -3: Acid - Metal Limiting Reactant - I • 2 Al(s) + 6 HCl(g) 2 Al. Cl 3(s) + 3 H 2(g) Consider the reaction above. If we react 30. 0 g Al and 20. 0 g HCl, how many moles of aluminum chloride will be formed? • 30. 0 g Al • 20. 0 g HCl • Limiting reactant = reactant with RRmin =

Problem 9 -3: Acid - Metal Limiting Reactant - II Calculate the yield of Al. Cl 3 from: Moles Al. Cl 3 = (RR)min x reaction coefficient of Al. Cl 3.

Problem 9 -4: Ostwald Process Limiting Reactant What mass of NO could be formed by the reaction 30. 0 g of ammonia gas and 40. 0 g of oxygen gas w/ the rxn below? 4 NH 3 (g) + 5 O 2 (g) 4 NO(g) + 6 H 2 O(g) 30. 0 g NH 3 40. 0 g O 2 RRmin =_______, therefore ______ is the Limiting Reactant! Moles NO formed = Mass NO =


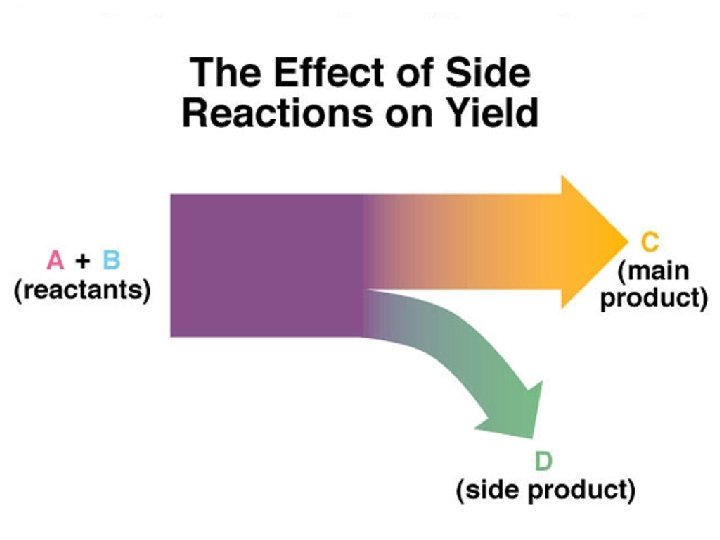
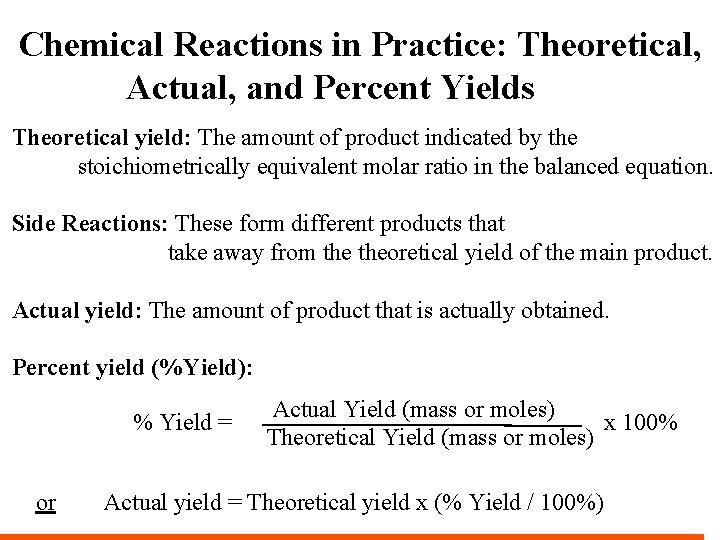
Chemical Reactions in Practice: Theoretical, Actual, and Percent Yields Theoretical yield: The amount of product indicated by the stoichiometrically equivalent molar ratio in the balanced equation. Side Reactions: These form different products that take away from theoretical yield of the main product. Actual yield: The amount of product that is actually obtained. Percent yield (%Yield): % Yield = or Actual Yield (mass or moles) x 100% Theoretical Yield (mass or moles) Actual yield = Theoretical yield x (% Yield / 100%)

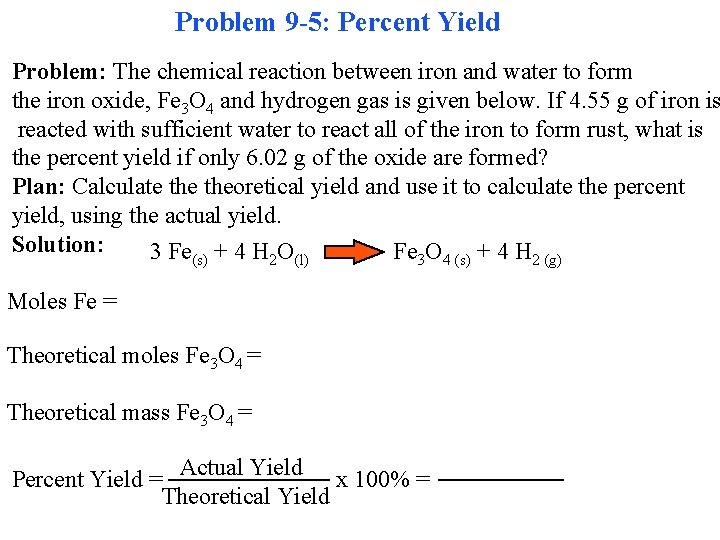
Problem 9 -5: Percent Yield Problem: The chemical reaction between iron and water to form the iron oxide, Fe 3 O 4 and hydrogen gas is given below. If 4. 55 g of iron is reacted with sufficient water to react all of the iron to form rust, what is the percent yield if only 6. 02 g of the oxide are formed? Plan: Calculate theoretical yield and use it to calculate the percent yield, using the actual yield. Solution: 3 Fe(s) + 4 H 2 O(l) Fe 3 O 4 (s) + 4 H 2 (g) Moles Fe = Theoretical moles Fe 3 O 4 = Theoretical mass Fe 3 O 4 = Actual Yield Percent Yield = x 100% = Theoretical Yield

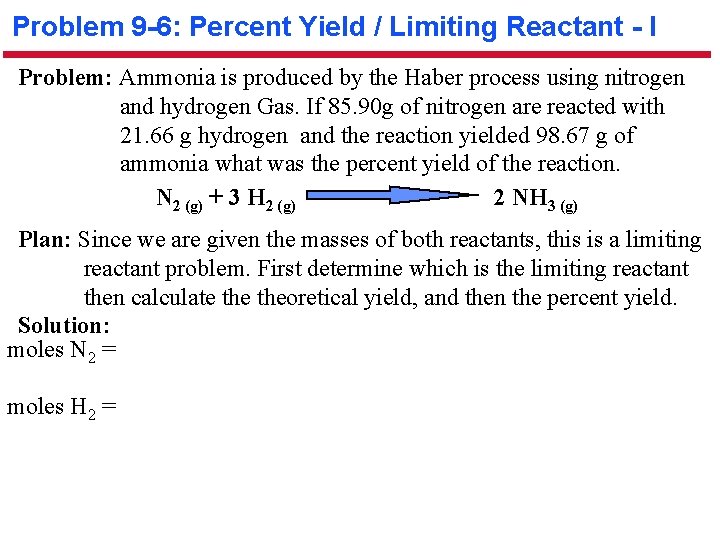
Problem 9 -6: Percent Yield / Limiting Reactant - I Problem: Ammonia is produced by the Haber process using nitrogen and hydrogen Gas. If 85. 90 g of nitrogen are reacted with 21. 66 g hydrogen and the reaction yielded 98. 67 g of ammonia what was the percent yield of the reaction. N 2 (g) + 3 H 2 (g) 2 NH 3 (g) Plan: Since we are given the masses of both reactants, this is a limiting reactant problem. First determine which is the limiting reactant then calculate theoretical yield, and then the percent yield. Solution: moles N 2 = moles H 2 =

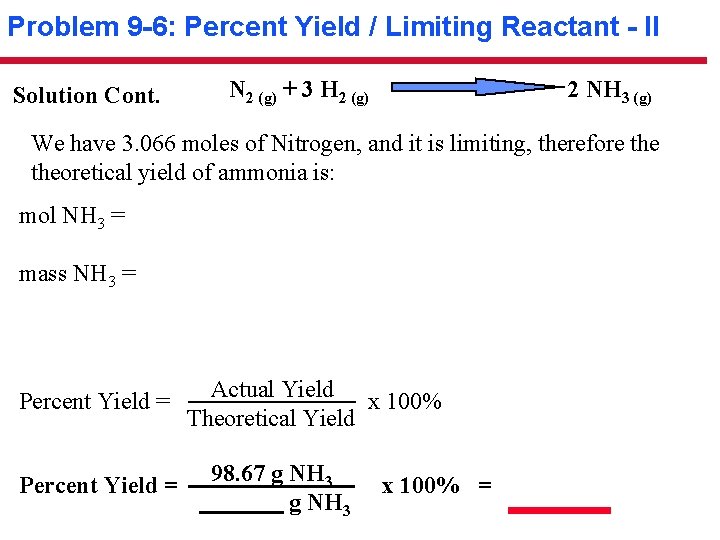
Problem 9 -6: Percent Yield / Limiting Reactant - II Solution Cont. N 2 (g) + 3 H 2 (g) 2 NH 3 (g) We have 3. 066 moles of Nitrogen, and it is limiting, therefore theoretical yield of ammonia is: mol NH 3 = mass NH 3 = Percent Yield = Actual Yield x 100% Theoretical Yield 98. 67 g NH 3 x 100% =

Answers to Problems in Lecture # 9 1. (a) 2. 629 moles H 2 O; (b) 44. 81 g H 2 S, 68. 36 g Al(OH)3 2. 77. 61 g Ca 3(PO 4)2 3. 0. 183 mol of Al. Cl 3 4. 30. 0 g NO 5. 95. 6 % 6. 94. 49 %