Lecture 7 Water and water vapor in the


![Vapor Pressure of Water Psat = A exp [B( 1/273. 15 – 1/T)] A=6. Vapor Pressure of Water Psat = A exp [B( 1/273. 15 – 1/T)] A=6.](https://slidetodoc.com/presentation_image/5e48ce99443506543b268f00f38732b3/image-5.jpg)








- Slides: 25

Lecture 7. Water and water vapor in the atmosphere 30 Sep 2010 • Review of buoyancy, with an unusual demonstration of Archimedes principle. • Water is a polar molecule that forms hydrogen bonds. Consequently water is a structured liquid (and solid!). Water has a very high latent heat for evaporation and fusion, due to the forces between molecules associated with hydrogen bonds (2. 5 x 106 J/kg). Ice is highly ordered, less dense than liquid water (very unusual), and has significant latent heat of fusion (0. 34 x 106 J/kg). • Evaporation and condensation are dynamic processes always taking place at the liquid-air interface. The rate of evaporation increases with temperature. When the rate of evaporation equals the rate of condensation, the air and water are in equilibrium, and the air is said to be saturated with water vapor. The relationship between water vapor pressure and temperature is the Clausius-Clapeyron equation. Psat = A exp [B( 1/273. 15 – 1/T)] B= 5308 K. A=6. 11 mbar=vapor press. 0 C. • Vapor pressure increases sharply with temperature, due to the large latent heat. Water vapor content of air may be reported as partial pressure, relative humidity, dew point or frost point, or specific humidity. In general water vapor content is smaller than Psat, never significantly greater. • Discuss the observed distribution of temperature in the atmosphere. • When an air parcel moves up or down, its pressure changes according to the barometric law. Forces act on the parcel and change its size, meaning that work is done on/by the parcel. Work done on an air parcel by atmosphere, or by the parcel on the atmosphere, is related to change in the temperature of the air parcel.

+ O H (—) H O + H H (—) One side of the water molecule has a negative electric charge, balanced by a positive charge on the other side. Water is a "polar" molecule. The positive side of each water molecule interacts strongly with the negative side of other water molecules. Water makes "hydrogen bonds", in ice, liquid, and vapor. A great deal of energy is needed to pull a water molecule out of the liquid, because of the strong hydrogen bonds. 2. 26 × 106 J/kg needed to evaporate water. For comparison: 4. 2 × 103 J/kg needed to raise the temperature of water by 1 K (1 C). The amount of energy needed to evaporate 1 kg of water is called the latent heat of vaporization. It is much larger for water than for most other liquids (due to hydrogen bonding).

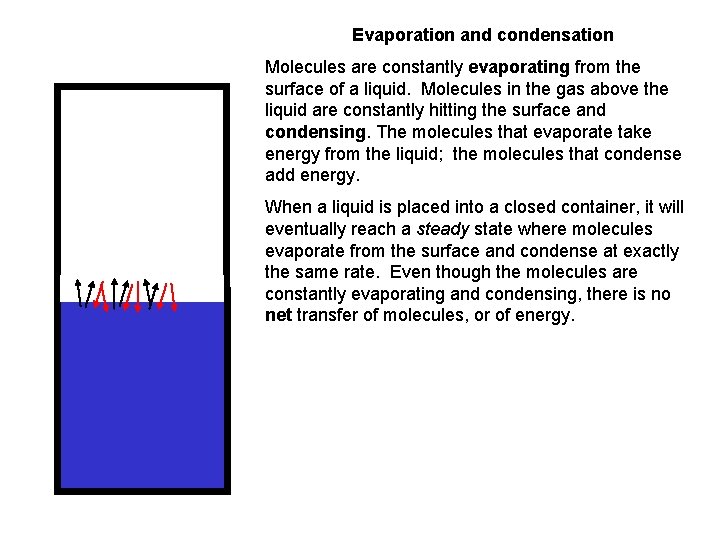
Evaporation and condensation Molecules are constantly evaporating from the surface of a liquid. Molecules in the gas above the liquid are constantly hitting the surface and condensing. The molecules that evaporate take energy from the liquid; the molecules that condense add energy. When a liquid is placed into a closed container, it will eventually reach a steady state where molecules evaporate from the surface and condense at exactly the same rate. Even though the molecules are constantly evaporating and condensing, there is no net transfer of molecules, or of energy.

Lecture 7. Water and water vapor in the atmosphere • Review of buoyancy, with an unusual demonstration of Archimedes principle. • Water is a polar molecule that forms hydrogen bonds. Consequently water is a structured liquid (and solid!). • Evaporation and condensation are dynamic processes always taking place at the liquid-air interface. The rate of evaporation increases with temperature. • When the rate of evaporation equals the rate of condensation, the air and water are in equilibrium, and the air is said to be saturated with water vapor. The relationship between water vapor pressure and temperature is the Clausius-Clapeyron equation. • Water has a very high latent heat for evaporation and fusion, due to the forces between molecules associated with hydrogen bonds (2. 5 x 106 J/kg). Thus the Clausius-Clapeyron equation shows a steep increase in vapor pressure with temperature: Psat = A exp [B( 1/273. 15 – 1/T)] B= 5308 K. A=6. 11 mbar=water vapor press. at 0 C. • Ice is highly ordered, less dense than liquid water (very unusual), and has significant latent heat of fusion (0. 34 x 106 J/kg). • Water vapor content of air may be reported as partial pressure, relative humidity, dew point or frost point, or specific humidity. In general water vapor content is smaller than Psat, never greater.
![Vapor Pressure of Water Psat A exp B 1273 15 1T A6 Vapor Pressure of Water Psat = A exp [B( 1/273. 15 – 1/T)] A=6.](https://slidetodoc.com/presentation_image/5e48ce99443506543b268f00f38732b3/image-5.jpg)
Vapor Pressure of Water Psat = A exp [B( 1/273. 15 – 1/T)] A=6. 11 mbar, B= 5308 K. A=water vapor pressure at 0 C. The pressure of H 2 O vapor in equilibrium with liquid water. Clausius. Clapeyron relation. Water vapor pressure versus T.

2253 k. J required for latent heat of evaporation– 1 kg 335 k. J required for latent heat of fusion 6

Lecture 7. Water and water vapor in the atmosphere • Review of buoyancy, with an unusual demonstration of Archimedes principle. • Water is a polar molecule that forms hydrogen bonds. Consequently water is a structured liquid (and solid!). • Evaporation and condensation are dynamic processes always taking place at the liquid-air interface. The rate of evaporation increases with temperature. • When the rate of evaporation equals the rate of condensation, the air and water are in equilibrium, and the air is said to be saturated with water vapor. The relationship between water vapor pressure and temperature is the Clausius-Clapeyron equation. • Water has a very high latent heat for evaporation and fusion, due to the forces between molecules associated with hydrogen bonds (2. 5 x 106 J/kg). Thus the Clausius-Clapeyron equation shows a steep increase in vapor pressure with temperature: Psat = A exp [B( 1/273. 15 – 1/T)] B= 5308 K. A=6. 11 mbar=water vapor press. at 0 C. • Ice is highly ordered, less dense than liquid water (very unusual), and has significant latent heat of fusion (0. 34 x 106 J/kg). • Water vapor content of air may be reported as partial pressure, relative humidity, dew point or frost point, or specific humidity. In general water vapor content is smaller than Psat, never greater.

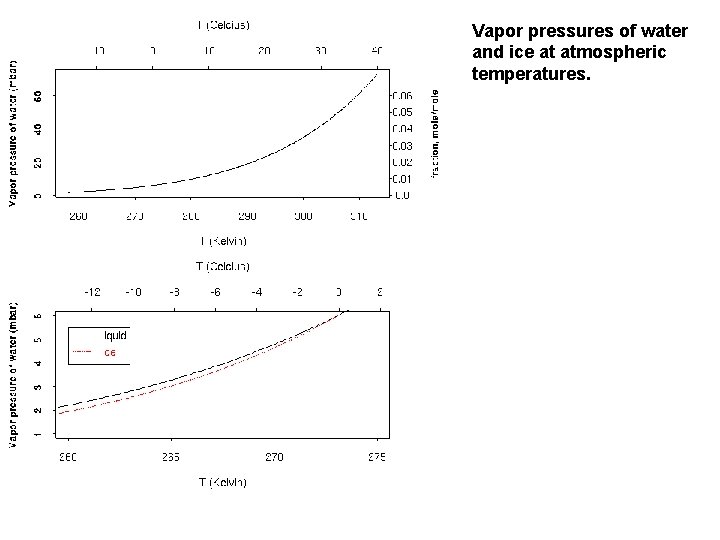
Vapor pressures of water and ice at atmospheric temperatures.

Measuring the water vapor content of the atmosphere. Relative humidity—air has a given amount of water vapor (partial pressure P, mb). In general it will have less water vapor than in equilibrium with liquid at the same T. Relative Humidity: Pwater/Psaturated (x 100, %) Dew point: Temperature to which you would have to cool the air to have 100% humidity (liquid condensation starts). Frost point: ice condenses. Specific Humidity: kg of H 2 O / kg of air NOTE: "supercooling of droplets"

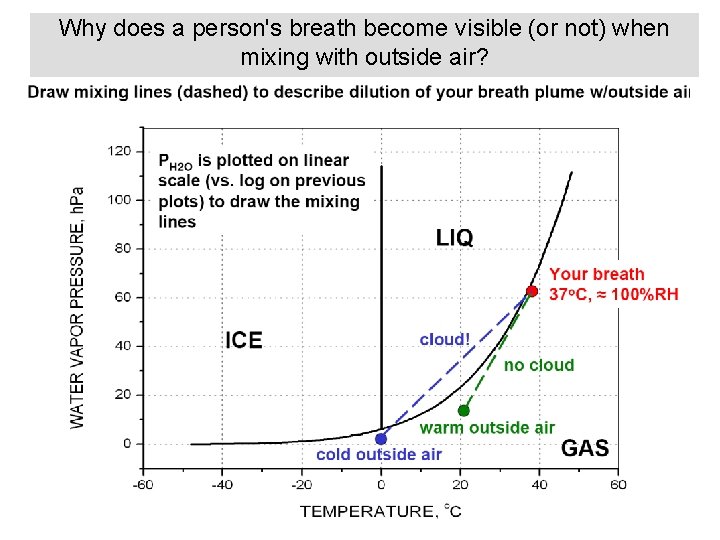
Why does a person's breath become visible (or not) when mixing with outside air?

Lecture 7. Water and water vapor in the atmosphere • Review of buoyancy, with an unusual demonstration of Archimedes principle. • Water is a polar molecule that forms hydrogen bonds. Consequently water is a structured liquid (and solid!). • Evaporation and condensation are dynamic processes always taking place at the liquid-air interface. The rate of evaporation increases with temperature. • When the rate of evaporation equals the rate of condensation, the air and water are in equilibrium, and the air is said to be saturated with water vapor. The relationship between water vapor pressure and temperature is the Clausius-Clapeyron equation. • Water has a very high latent heat for evaporation and fusion, due to the forces between molecules associated with hydrogen bonds (2. 5 x 106 J/kg). Thus the Clausius-Clapeyron equation shows a steep increase in vapor pressure with temperature: Psat = A exp [B( 1/273. 15 – 1/T)] B= 5308 K. A=6. 11 mbar=water vapor press. at 0 C. • Ice is highly ordered, less dense than liquid water (very unusual), and has significant latent heat of fusion (0. 34 x 106 J/kg). • Water vapor content of air may be reported as partial pressure, relative humidity, dew point or frost point, or specific humidity. In general water vapor content is smaller than Psat, never greater. • Introduce atmospheric temperature regions.

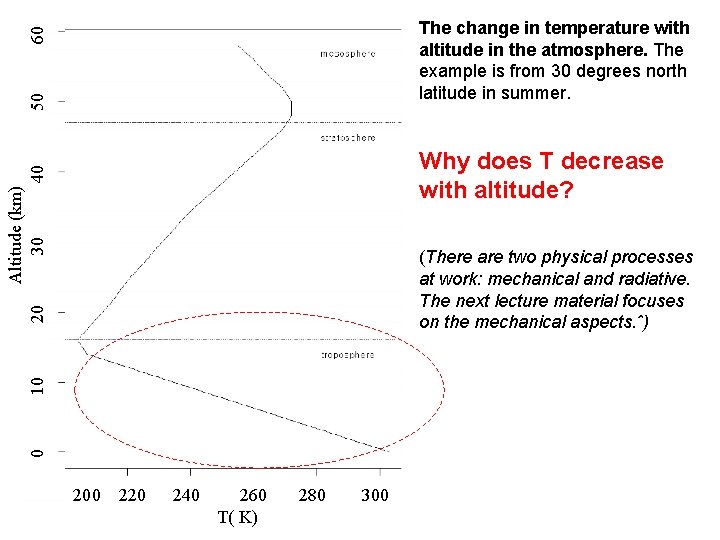
50 60 The change in temperature with altitude in the atmosphere. The example is from 30 degrees north latitude in summer. Altitude (km) 30 40 Why does T decrease with altitude? 0 10 20 (There are two physical processes at work: mechanical and radiative. The next lecture material focuses on the mechanical aspects. ˆ) 200 220 240 260 T( K) 280 300

The concept of an air parcel 1) It's a distinct 'block' of air in an environment of … air; we often assume it has volume of 1 m 3. It has to be small enough so that it has uniform properties (T, P, etc). It’s a fictional entity that helps us to think through a physical process. 2) We can follow it (as if it were colored with dye) and it stays together (the same molecules are inside at the end of a process as there originally). 3) At the beginning of any of thought exercise, it has the same characteristics as its surrounding environment. 4) The parcel can change with time, by moving, emitting or absorbing heat radiation, etc --usually in a way we can describe with equations. 5) The environment of the parcel can change too. The parcel changes as a parcel NOT necessarily with the environment.

Question: Where does the energy come from for an air parcel to do this work on the atmosphere?

Change of atmospheric temperature with altitude ( pressure ) Atmospheric pressure vs altitude follows the barometric law, ΔP=-ρgΔz. Let's think of an ideal case where the buoyancy forces and the weight of an air parcel are perfectly balanced at every altitude, and we neither add or remove heat as the parcel moves. Because an air parcel expands as pressure is lowered, it must do work on the atmosphere as it moves up. The only source of energy is the motion of the molecules, and therefore the air parcel must get colder as it moves up. Two steps are needed to understand how an air parcel that moves up or down changes it temperature. Step 1. Figure out the exchanges of energy between the air parcel and the environment as the parcel changes its pressure, using the definition of heat capacity and Boyle's law. Step 2. Relate this energy balance to the change in altitude, using the barometric law.

Boyle's Law: P 1 V 1 = P 2 V 2 How can we use Boyle's Law to determine the change in V when P changes, for a parcel of air (at constant temperature)? Boyles Law: P 2 V 2 = P 1 V 1 + P 1ΔV + V 1ΔP + ΔPΔV = P 1 V 1 V (P 1 + ΔP 1)( V 1 + ΔV 1) = P 2 V 2 Boyles law P 1ΔV = — V 1ΔP, or ΔP/ΔV = — P 1/V 1 P P 1+ΔP 1 = P 2 ; V 1 + ΔV 1 = V 2 This is an example of how we can understand the relationship between two properties of air (or any gas), when both change together, by dividing the process into very small steps where one changes while the other is held constant, then hold the first constant and change the one initially held fixed. ΔV/V 1 = ─ ΔP/P 1

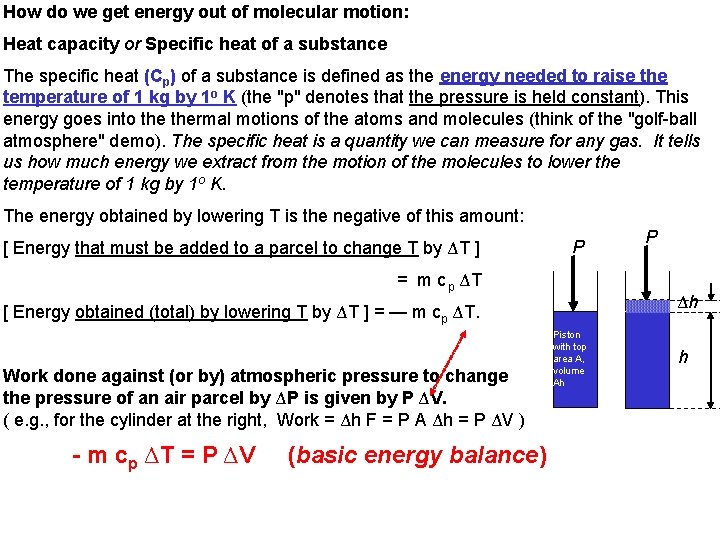
How do we get energy out of molecular motion: Heat capacity or Specific heat of a substance The specific heat (Cp) of a substance is defined as the energy needed to raise the temperature of 1 kg by 1 o K (the "p" denotes that the pressure is held constant). This energy goes into thermal motions of the atoms and molecules (think of the "golf-ball atmosphere" demo). The specific heat is a quantity we can measure for any gas. It tells us how much energy we extract from the motion of the molecules to lower the temperature of 1 kg by 1 o K. The energy obtained by lowering T is the negative of this amount: [ Energy that must be added to a parcel to change T by ΔT ] P = m c p ΔT Δh [ Energy obtained (total) by lowering T by ΔT ] = — m cp ΔT. Work done against (or by) atmospheric pressure to change the pressure of an air parcel by ΔP is given by P ΔV. ( e. g. , for the cylinder at the right, Work = Δh F = P A Δh = P ΔV ) - m cp ΔT = P ΔV (basic energy balance) P Piston with top area A, volume Ah h

- mcp ΔT = P ΔV (basic energy balance) VΔP = - P ΔV (Boyle’s law) =>> - mcp ΔT = - VΔP ΔP = - g ρ ΔZ (Barometric law) =>>- mc p ΔT = (Vρ) g ΔZ ρV = mass of parcel We see that for an air parcel moving vertically in a hydrostatic atmosphere (barometric law applies), - cp ΔT = g ΔZ ΔT / Δz = -g/cp = - 9. 8 o. K/km This change in temperature with altitude is called the "adiabatic lapse rate". cp = 1005 J/kg/K; g = 9. 8 m s-2 =>> - g / cp = — 9. 8 x 10 -3 K/m or — 9. 8 K/km.

Lecture 7. Water and water vapor in the atmosphere 30 Sep 2010 • Review of buoyancy, with an unusual demonstration of Archimedes principle. • Water is a polar molecule that forms hydrogen bonds. Consequently water is a structured liquid (and solid!). Water has a very high latent heat for evaporation and fusion, due to the forces between molecules associated with hydrogen bonds (2. 5 x 106 J/kg). Ice is highly ordered, less dense than liquid water (very unusual), and has significant latent heat of fusion (0. 34 x 106 J/kg). • Evaporation and condensation are dynamic processes always taking place at the liquid-air interface. The rate of evaporation increases with temperature. When the rate of evaporation equals the rate of condensation, the air and water are in equilibrium, and the air is said to be saturated with water vapor. The relationship between water vapor pressure and temperature is the Clausius-Clapeyron equation. Psat = A exp [B( 1/273. 15 – 1/T)] B= 5308 K. A=6. 11 mbar=vapor press. 0 o. C. • Vapor pressure increases sharply with temperature, due to the large latent heat. Water vapor content of air may be reported as partial pressure, relative humidity, dew point or frost point, or specific humidity. In general water vapor content is smaller than Psat, never significantly greater. • Discuss the observed distribution of temperature in the atmosphere. • When an air parcel moves up or down, its pressure changes according to the barometric law. Forces act on the parcel and change its size, meaning that work is done on/by the parcel. Work done on an air parcel by atmosphere, or by the parcel on the atmosphere, is related to change in the temperature of the air parcel.

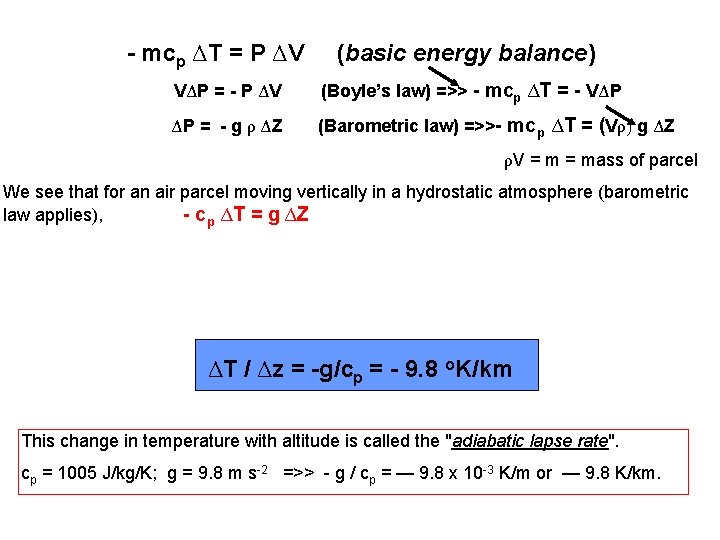
Global Sea Surface Temperatures February 2002

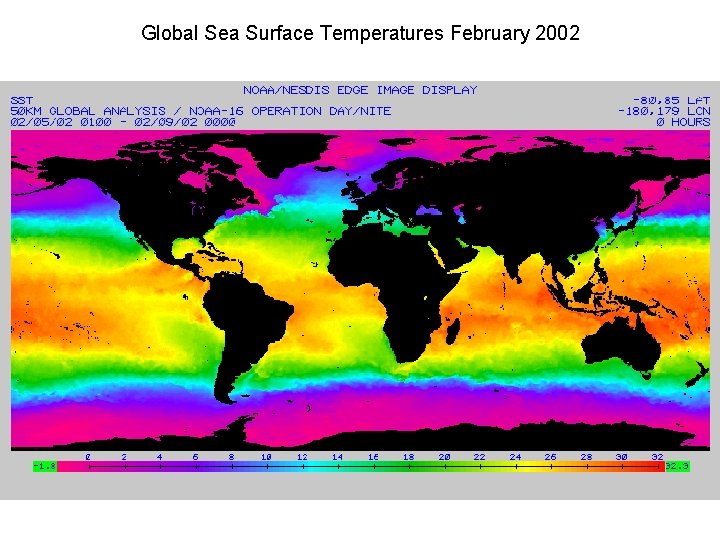
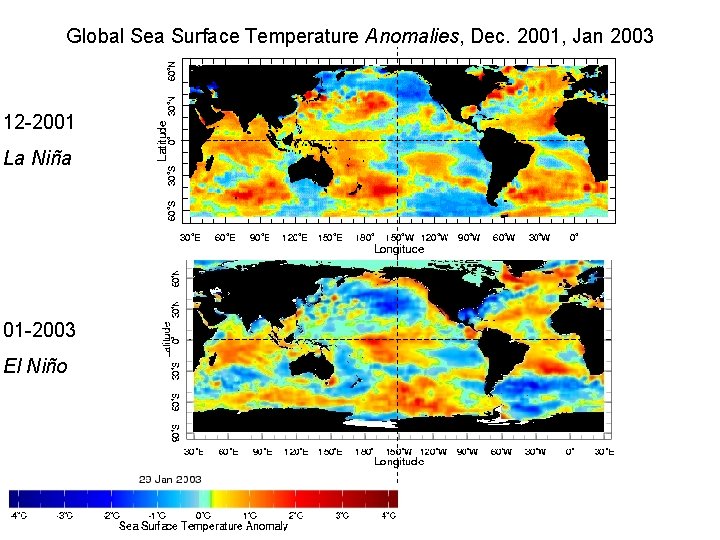
Global Sea Surface Temperature Anomalies, December 2001

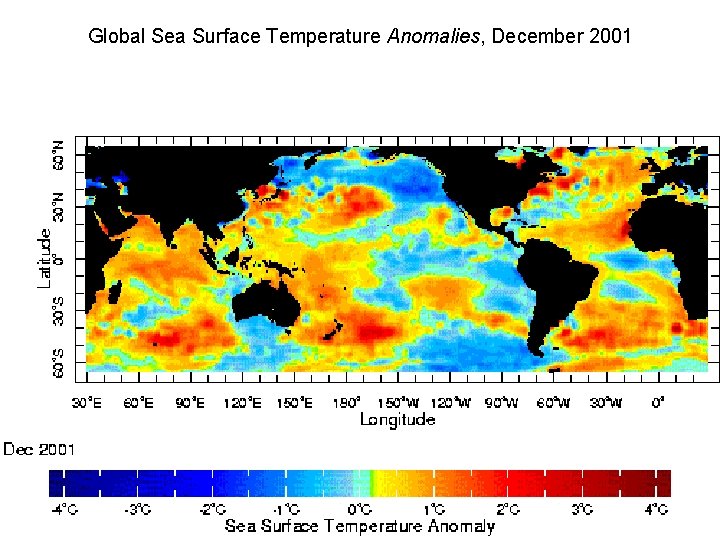
10 Feb 2002 GOES ir image

10 Feb 2002 GOES ir image

/www. cira. colostate. edu/Special/Curr. Wx/g 8 full 40. asp Feb 2003

Global Sea Surface Temperature Anomalies, Dec. 2001, Jan 2003 12 -2001 La Niña 01 -2003 El Niño
 Superaquecimento
Superaquecimento Water and water and water water
Water and water and water water Why is earth called the blue planet
Why is earth called the blue planet 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Clausius clapeyron equation
Clausius clapeyron equation Stomatal transpiration diagram
Stomatal transpiration diagram The capacity of air to hold water vapor
The capacity of air to hold water vapor Vapor pressure of water equation
Vapor pressure of water equation Water vapor in the atmosphere percentage
Water vapor in the atmosphere percentage Water vapor mixing ratio
Water vapor mixing ratio Raoult's law and dalton's law
Raoult's law and dalton's law Water vapor gif
Water vapor gif Chemical equation for water vapor
Chemical equation for water vapor To beat rapidly to incorporate air and increase volume
To beat rapidly to incorporate air and increase volume Vapor pressure worksheet
Vapor pressure worksheet Induced dipole induced dipole attraction
Induced dipole induced dipole attraction Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Tư thế worm breton
Tư thế worm breton Alleluia hat len nguoi oi
Alleluia hat len nguoi oi Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới